Deck 28: Quantum Mechanics of Atoms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 28: Quantum Mechanics of Atoms
1
Choose the one alternative that best completes the statement or answers the question.
The principal quantum number n can have any integer value ranging from
The principal quantum number n can have any integer value ranging from

D
2
Choose the one alternative that best completes the statement or answers the question.
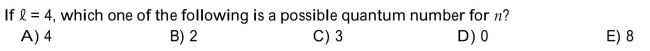
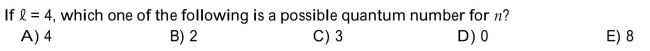
E
3
Write the word or phrase that best completes each statement or answers the question.



4
Choose the one alternative that best completes the statement or answers the question.
If the maximum possible accuracy in measuring the velocity of a particle increases, the maximum possible accuracy in measuring its position will
A) increase.
B) decrease.
C) not be affected.
If the maximum possible accuracy in measuring the velocity of a particle increases, the maximum possible accuracy in measuring its position will
A) increase.
B) decrease.
C) not be affected.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
Choose the one alternative that best completes the statement or answers the question.
The magnetic quantum number m1 can have any integer value ranging from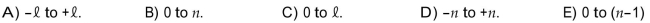
The magnetic quantum number m1 can have any integer value ranging from
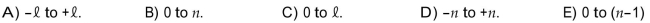

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
Choose the one alternative that best completes the statement or answers the question.
Which of the following values can be taken by the electron spin quantum number, ms?
A) ±3
B) 0
C) ±1
D) ±2
E) ±1/2
Which of the following values can be taken by the electron spin quantum number, ms?
A) ±3
B) 0
C) ±1
D) ±2
E) ±1/2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Choose the one alternative that best completes the statement or answers the question.
The orbital angular momentum quantum number can take which of the following values for any given value of the principal quantum number, n?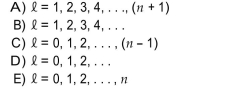
The orbital angular momentum quantum number can take which of the following values for any given value of the principal quantum number, n?
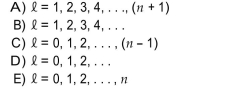

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
Choose the one alternative that best completes the statement or answers the question.
The electron spin quantum number can have values of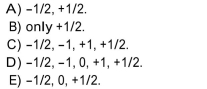
The electron spin quantum number can have values of
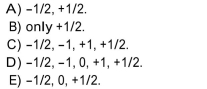

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
Choose the one alternative that best completes the statement or answers the question.
According to Pauli's exclusion principle, how many electrons in an atom may have a particular set of quantum numbers?
A) 4
B) 5
C) 2
D) 3
E) 1
According to Pauli's exclusion principle, how many electrons in an atom may have a particular set of quantum numbers?
A) 4
B) 5
C) 2
D) 3
E) 1

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
Choose the one alternative that best completes the statement or answers the question.
According to the quantum mechanical model of the hydrogen atom, if the principal quantum number is n, how many different orbital angular momentum quantum numbers are permitted?
A) 2n
B) 4n
C) 3n
D) n
E) n/2
According to the quantum mechanical model of the hydrogen atom, if the principal quantum number is n, how many different orbital angular momentum quantum numbers are permitted?
A) 2n
B) 4n
C) 3n
D) n
E) n/2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
Choose the one alternative that best completes the statement or answers the question.
If the maximum possible accuracy in measuring the lifetime of a particle increases, the maximum possible accuracy in measuring its energy will
A) increase.
B) decrease.
C) not be affected.
If the maximum possible accuracy in measuring the lifetime of a particle increases, the maximum possible accuracy in measuring its energy will
A) increase.
B) decrease.
C) not be affected.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
Choose the one alternative that best completes the statement or answers the question.
The orbital angular momentum quantum number ʆ can have any integer value ranging from
A) 0 to (n-1).
B) 0 to n.
C) 1 to (n+1).
D) 1 to n.
E) -n to n.
The orbital angular momentum quantum number ʆ can have any integer value ranging from
A) 0 to (n-1).
B) 0 to n.
C) 1 to (n+1).
D) 1 to n.
E) -n to n.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Choose the one alternative that best completes the statement or answers the question.
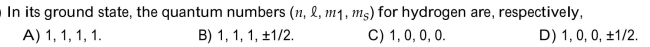
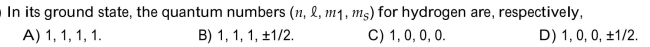

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Choose the one alternative that best completes the statement or answers the question.
A hydrogen atom is in the 6h state. What is the principal quantum number.
A) 6
B) 7
C) 5
D) 0
E) 3
A hydrogen atom is in the 6h state. What is the principal quantum number.
A) 6
B) 7
C) 5
D) 0
E) 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
Choose the one alternative that best completes the statement or answers the question.
If the maximum possible accuracy in measuring the energy of a particle increases, the maximum possible accuracy in measuring its lifetime will
A) increase.
B) decrease.
C) not be affected.
If the maximum possible accuracy in measuring the energy of a particle increases, the maximum possible accuracy in measuring its lifetime will
A) increase.
B) decrease.
C) not be affected.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
Choose the one alternative that best completes the statement or answers the question.
If the maximum possible accuracy in measuring the position of a particle increases, the maximum possible accuracy in measuring its velocity will
A) increase.
B) decrease.
C) not be affected.
If the maximum possible accuracy in measuring the position of a particle increases, the maximum possible accuracy in measuring its velocity will
A) increase.
B) decrease.
C) not be affected.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
Write the word or phrase that best completes each statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
Write the word or phrase that best completes each statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
Write the word or phrase that best completes each statement or answers the question.
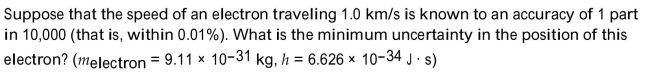
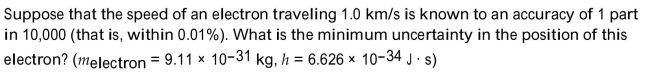

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
Write the word or phrase that best completes each statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
Write the word or phrase that best completes each statement or answers the question.
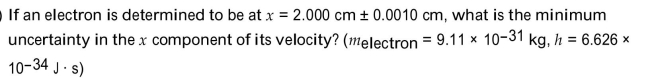
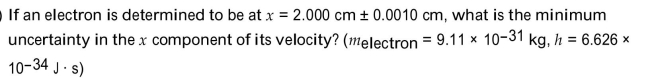

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Write the word or phrase that best completes each statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Choose the one alternative that best completes the statement or answers the question.
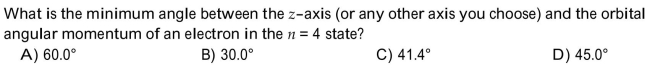
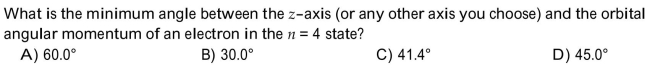

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
Choose the one alternative that best completes the statement or answers the question.
A hydrogen atom is in the 6h state. Which one of the following numbers could be an orbital angular momentum quantum number ʆ for that state?
A) 5
B) 6
C) 8
D) 9
E) 7
A hydrogen atom is in the 6h state. Which one of the following numbers could be an orbital angular momentum quantum number ʆ for that state?
A) 5
B) 6
C) 8
D) 9
E) 7

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
Choose the one alternative that best completes the statement or answers the question.
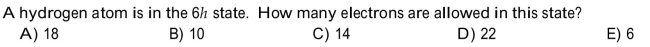
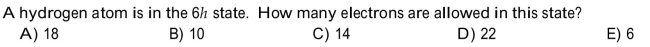

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Choose the one alternative that best completes the statement or answers the question.
In a hydrogen atom, a given electron has n = 7. How many possible values can ʆ have?
A) 6
B) 98
C) 7
D) 15
In a hydrogen atom, a given electron has n = 7. How many possible values can ʆ have?
A) 6
B) 98
C) 7
D) 15

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
Write the word or phrase that best completes each statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Choose the one alternative that best completes the statement or answers the question.
A hydrogen atom is in the 6h state. Which one of the following is not a magnetic quantum number for that state?
A) 2
B) 4
C) 1
D) 6
E) 0
A hydrogen atom is in the 6h state. Which one of the following is not a magnetic quantum number for that state?
A) 2
B) 4
C) 1
D) 6
E) 0

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
Choose the one alternative that best completes the statement or answers the question.
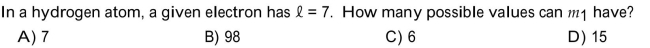
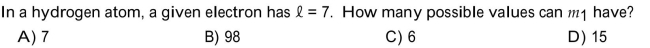

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
Write the word or phrase that best completes each statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
Write the word or phrase that best completes each statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
Choose the one alternative that best completes the statement or answers the question.
In a hydrogen atom, an electron with n = 7 can exist in how many different quantum states?
A) 6
B) 98
C) 7
D) 15
In a hydrogen atom, an electron with n = 7 can exist in how many different quantum states?
A) 6
B) 98
C) 7
D) 15

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Choose the one alternative that best completes the statement or answers the question.
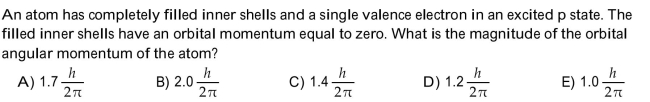
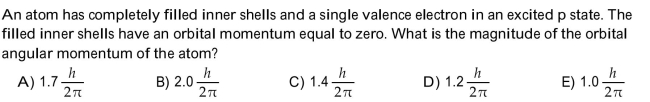

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
Choose the one alternative that best completes the statement or answers the question.
How many 2d electron states can an atom have?
A) 8
B) 4
C) 6
D) 10
E) 0
How many 2d electron states can an atom have?
A) 8
B) 4
C) 6
D) 10
E) 0

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
Choose the one alternative that best completes the statement or answers the question.
For the ground state of the hydrogen atom, which of the following numbers represents the correct value of the magnetic quantum number?
A) 1
B) -2
C) -1
D) 2
E) 0
For the ground state of the hydrogen atom, which of the following numbers represents the correct value of the magnetic quantum number?
A) 1
B) -2
C) -1
D) 2
E) 0

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
Choose the one alternative that best completes the statement or answers the question.
How many 3d electron states can an atom have?
A) 6
B) 8
C) 4
D) 10
E) 0
How many 3d electron states can an atom have?
A) 6
B) 8
C) 4
D) 10
E) 0

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
Choose the one alternative that best completes the statement or answers the question.
How many electrons can be found with principal quantum number n = 3 in a suitably heavy atom?
A) 18
B) 6
C) 9
D) 20
How many electrons can be found with principal quantum number n = 3 in a suitably heavy atom?
A) 18
B) 6
C) 9
D) 20

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Choose the one alternative that best completes the statement or answers the question.
How many unique quantum states correspond to the lowest possible energy level of an electron in the hydrogen atom?
A) 3
B) 1
C) 4
D) 0
E) 2
How many unique quantum states correspond to the lowest possible energy level of an electron in the hydrogen atom?
A) 3
B) 1
C) 4
D) 0
E) 2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Choose the one alternative that best completes the statement or answers the question.
How many values can the magnetic quantum number have in a hydrogen atom for which the orbital angular momentum quantum number is equal to 8?
A) 8
B) 5
C) 15
D) 9
E) 17
How many values can the magnetic quantum number have in a hydrogen atom for which the orbital angular momentum quantum number is equal to 8?
A) 8
B) 5
C) 15
D) 9
E) 17

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Choose the one alternative that best completes the statement or answers the question.
For the ground state of the hydrogen atom, which of the following numbers represents the correct value of the orbital angular momentum quantum number?
A) 1
B) -1
C) 0
D) 2
E) -2
For the ground state of the hydrogen atom, which of the following numbers represents the correct value of the orbital angular momentum quantum number?
A) 1
B) -1
C) 0
D) 2
E) -2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
Choose the one alternative that best completes the statement or answers the question.
How many values of the magnetic quantum number, mʆ, correspond to a value of ʆ = 4?
A) 7
B) 5
C) 9
D) 8
E) 3
How many values of the magnetic quantum number, mʆ, correspond to a value of ʆ = 4?
A) 7
B) 5
C) 9
D) 8
E) 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
Choose the one alternative that best completes the statement or answers the question.
How many electrons will fit into a 4f subshell?
A) 14
B) 7
C) 3
D) 4
How many electrons will fit into a 4f subshell?
A) 14
B) 7
C) 3
D) 4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
Choose the one alternative that best completes the statement or answers the question.



Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
Choose the one alternative that best completes the statement or answers the question.
How many possible sets of electron states (or quantum numbers) are there in the 5f subshell?
A) 14
B) 2
C) 8
D) 10
How many possible sets of electron states (or quantum numbers) are there in the 5f subshell?
A) 14
B) 2
C) 8
D) 10

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
Choose the one alternative that best completes the statement or answers the question.
What is the maximum number of electrons that can occupy the g subshell?
A) 18
B) 22
C) 14
D) 10
What is the maximum number of electrons that can occupy the g subshell?
A) 18
B) 22
C) 14
D) 10

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


