Deck 40: Molecules and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 40: Molecules and Solids
1
Covalent bonding is caused by
A)the sharing of electrons between atoms.
B)the transfer of electrons between atoms.
C)unequal charge distributions around neutral molecules.
D)atoms bonding to hydrogen molecules.
E)atoms bonding to oxygen molecules.
A)the sharing of electrons between atoms.
B)the transfer of electrons between atoms.
C)unequal charge distributions around neutral molecules.
D)atoms bonding to hydrogen molecules.
E)atoms bonding to oxygen molecules.
the sharing of electrons between atoms.
2
In a good conductor, the highest energy band containing electrons is
A)only partially filled.
B)completely filled.
A)only partially filled.
B)completely filled.
only partially filled.
3
Metallic bonding is caused by
A)the sharing of electrons by all atoms.
B)the transfer of electrons between atoms.
C)unequal charge distributions around neutral molecules.
D)atoms bonding to hydrogen molecules.
E)atoms bonding to oxygen molecules.
A)the sharing of electrons by all atoms.
B)the transfer of electrons between atoms.
C)unequal charge distributions around neutral molecules.
D)atoms bonding to hydrogen molecules.
E)atoms bonding to oxygen molecules.
the sharing of electrons by all atoms.
4
The ionic bond forms because the atoms involved
A)are naturally ions.
B)have spherically symmetric charge distributions.
C)are the same.
D)all of the above
E)none of the above
A)are naturally ions.
B)have spherically symmetric charge distributions.
C)are the same.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
The binding energy of a molecule
A)is the negative of the activation energy.
B)cannot be negative if a molecule is formed.
C)must be negative if a molecule is formed.
D)can be negative if a molecule is formed.
E)must be positive if the molecule is formed.
A)is the negative of the activation energy.
B)cannot be negative if a molecule is formed.
C)must be negative if a molecule is formed.
D)can be negative if a molecule is formed.
E)must be positive if the molecule is formed.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
A p-type semiconductor has a net positive charge.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
For a diatomic quantum mechanical vibrator, the energy difference between adjacent quantum states
A)increases as v increases.
B)decreases as v increases.
C)is constant for all values of v.
D)varies randomly as v increases.
A)increases as v increases.
B)decreases as v increases.
C)is constant for all values of v.
D)varies randomly as v increases.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
A semiconductor can conduct electricity
A)only below room temperature.
B)never.
C)only very much above room temperature.
D)at room temperature but conductivity increases with increasing temperature.
E)at room temperature but conductivity decreases with increasing temperature.
A)only below room temperature.
B)never.
C)only very much above room temperature.
D)at room temperature but conductivity increases with increasing temperature.
E)at room temperature but conductivity decreases with increasing temperature.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
In an insulator, the valence band is
A)half filled.
B)more than half filled.
C)less than half filled.
D)separated from the conduction band by ~5 or more eV.
E)separated from the conduction band by ~1 or less eV.
A)half filled.
B)more than half filled.
C)less than half filled.
D)separated from the conduction band by ~5 or more eV.
E)separated from the conduction band by ~1 or less eV.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
A diatomic quantum mechanical rotator in the L = 1 quantum state has energy E. The same rotator in the L = 2 quantum state will have energy
A)2E.
B)3E.
C)6E.
D)8E.
E)none of the given answers
A)2E.
B)3E.
C)6E.
D)8E.
E)none of the given answers

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
In its lowest quantum state, the energy of a diatomic harmonic oscillator is
A)(1/4)hf.
B)(1/2)hf.
C)hf.
D)2hf.
E)(3/2)hf.
A)(1/4)hf.
B)(1/2)hf.
C)hf.
D)2hf.
E)(3/2)hf.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
Ionic bonding is caused by
A)the sharing of electrons between atoms.
B)the transfer of electrons between atoms.
C)unequal charge distributions around neutral molecules.
D)atoms bonding to hydrogen molecules.
E)atoms bonding to oxygen molecules.
A)the sharing of electrons between atoms.
B)the transfer of electrons between atoms.
C)unequal charge distributions around neutral molecules.
D)atoms bonding to hydrogen molecules.
E)atoms bonding to oxygen molecules.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
For a diatomic quantum mechanical rotator, the energy difference between adjacent energy levels
A)increases as L increases.
B)decreases as L increases.
C)is constant for all L.
D)varies randomly as L increases.
A)increases as L increases.
B)decreases as L increases.
C)is constant for all L.
D)varies randomly as L increases.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
A diatomic quantum mechanical vibrator in its ground state has energy E. This same vibrator in its third state has energy
A)E.
B)3E.
C)5E.
D)7E.
E)9E.
A)E.
B)3E.
C)5E.
D)7E.
E)9E.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
In the potential energy diagram of a molecule, the activation energy is the energy that is
A)necessary to get the molecule to form.
B)above the ground state of the molecule.
C)released when the molecule is formed.
D)stored in the molecule.
E)at the ground state of the molecule.
A)necessary to get the molecule to form.
B)above the ground state of the molecule.
C)released when the molecule is formed.
D)stored in the molecule.
E)at the ground state of the molecule.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
Van der Waals bonding is caused by
A)the sharing of electrons between atoms.
B)the transfer of electrons between atoms.
C)unequal charge distribution around neutral molecules.
D)atoms bonding to hydrogen molecules.
E)atoms bonding to oxygen molecules.
A)the sharing of electrons between atoms.
B)the transfer of electrons between atoms.
C)unequal charge distribution around neutral molecules.
D)atoms bonding to hydrogen molecules.
E)atoms bonding to oxygen molecules.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
If the potential energy diagram, when shown as a function of the separation of the atoms in the molecule, has a positive slope, then the force between the atoms
A)has the highest possible value.
B)is attractive.
C)has the lowest possible value.
D)is repulsive.
E)is none of the above.
A)has the highest possible value.
B)is attractive.
C)has the lowest possible value.
D)is repulsive.
E)is none of the above.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
In its lowest quantum state, a diatomic quantum mechanical rotator has a rotational energy of
A)zero.
B)2h/πI.
C)h/2πI.
D)h/πI.
E)none of the given answers
A)zero.
B)2h/πI.
C)h/2πI.
D)h/πI.
E)none of the given answers

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
The bond energy for a hydrogen molecule is
A)1.5 eV.
B)2.5 eV.
C)3.5 eV.
D)4.5 eV.
E)5.5 eV.
A)1.5 eV.
B)2.5 eV.
C)3.5 eV.
D)4.5 eV.
E)5.5 eV.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
In a good insulator, the highest energy band containing electrons, called the valence band, is
A)only partially filled.
B)completely filled.
A)only partially filled.
B)completely filled.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
A diatomic quantum mechanical oscillator has a moment of inertia of 7.73 × 10-45 kg∙m2. What is the rotational energy in the quantum state characterized by L = 2?
A)2.27 × 10-5 eV
B)2.72 × 10-5 eV
C)5.87 × 10-5 eV
D)7.22 × 10-5 eV
E)8.71 × 10-5 eV
A)2.27 × 10-5 eV
B)2.72 × 10-5 eV
C)5.87 × 10-5 eV
D)7.22 × 10-5 eV
E)8.71 × 10-5 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
In a p-type semiconductor, a hole is
A)a donor atom.
B)an extra electron supplied by a donor atom.
C)an extra proton supplied by a donor atom.
D)a missing atom in the crystalline structure.
E)a region where an electron is missing.
A)a donor atom.
B)an extra electron supplied by a donor atom.
C)an extra proton supplied by a donor atom.
D)a missing atom in the crystalline structure.
E)a region where an electron is missing.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is true?
A)In a p-type doped semiconductor the donor level is close to the valence band.
B)In an n-type doped semiconductor the acceptor level is close to the valence band.
C)In a p-type doped semiconductor the acceptor level is close to the valence band.
D)In an n-type doped semiconductor the donor level is close to the valence band.
E)None of the statements are true.
A)In a p-type doped semiconductor the donor level is close to the valence band.
B)In an n-type doped semiconductor the acceptor level is close to the valence band.
C)In a p-type doped semiconductor the acceptor level is close to the valence band.
D)In an n-type doped semiconductor the donor level is close to the valence band.
E)None of the statements are true.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
The spacing of the atoms in the H2 molecule is 7.4 x 10-11 m. What is the moment of inertia of this molecule about its center of mass?
A)1.5 x 10-47 kg∙ m2
B)1.9 x 10-47 kg∙ m2
C)9.1 x 10-48 kg∙ m2
D)4.6 x 10-48 kg∙ m2
E)2.3 x 10-48 kg∙v
A)1.5 x 10-47 kg∙ m2
B)1.9 x 10-47 kg∙ m2
C)9.1 x 10-48 kg∙ m2
D)4.6 x 10-48 kg∙ m2
E)2.3 x 10-48 kg∙v

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
A diatomic molecule is vibrating in the v = 1 quantum state with a frequency of 2.0 × 1013 Hz. What is the energy of vibration?
A)0.041 eV
B)0.083 eV
C)0.12 eV
D)0.15 eV
E)0.17 eV
A)0.041 eV
B)0.083 eV
C)0.12 eV
D)0.15 eV
E)0.17 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
A p-type semiconductor is produced by
A)doping the host crystal with donor impurities.
B)doping the host crystal with accepter impurities.
C)pure crystals of germanium.
D)all of the above.
E)none of the above.
A)doping the host crystal with donor impurities.
B)doping the host crystal with accepter impurities.
C)pure crystals of germanium.
D)all of the above.
E)none of the above.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
The characteristic rotational energy, h2/8π2I, for O2 is 2.9 × 10-23 J. Calculate the O2 bond length.
A)3.6 × 10-10 m
B)0.60 × 10-11 m
C)1.2 × 10-10 m
D)0.30 × 10-11 m
E)2.4 × 10-10 m
A)3.6 × 10-10 m
B)0.60 × 10-11 m
C)1.2 × 10-10 m
D)0.30 × 10-11 m
E)2.4 × 10-10 m

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
When a voltage is applied across a p-type semiconductor, the holes
A)are increased in number.
B)are destroyed.
C)move toward the positive electrode.
D)move toward the negative electrode.
E)do not move.
A)are increased in number.
B)are destroyed.
C)move toward the positive electrode.
D)move toward the negative electrode.
E)do not move.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
When a p-n junction diode is forward biased
A)a current does not flow through it.
B)a current flows through it from the p-side to the n-side.
C)a current flows through it from the n-side to the p-side.
D)a current flows because holes and electrons are traveling in opposite directions away from the junction.
E)a current flows because holes and electrons are traveling in the same direction towards the junction.
A)a current does not flow through it.
B)a current flows through it from the p-side to the n-side.
C)a current flows through it from the n-side to the p-side.
D)a current flows because holes and electrons are traveling in opposite directions away from the junction.
E)a current flows because holes and electrons are traveling in the same direction towards the junction.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
If a battery is connected to a diode with the positive terminal to the p side and the negative terminal to the n side, then diode is said to be
A)forward biased.
B)reversed biased.
A)forward biased.
B)reversed biased.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
A simple junction transistor consists of three semiconductor sections consisting of
A)pnp semiconductors.
B)npn semiconductors.
C)either pnp or npn semiconductors.
D)none of the given answers
A)pnp semiconductors.
B)npn semiconductors.
C)either pnp or npn semiconductors.
D)none of the given answers

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
The potential energy for the formation of a hydrogen molecule can be approximated by U = -A + BR + C/R, where R is the nuclear separation. Given that A = -78.5 eV, B = 2.57 × 1011 eV/m , and C = 1.44 × 10-9 eV∙m. At what nuclear separation is the potential energy a minimum?
A)1.9 × 10-11 m
B)3.7 × 10-10 m
C)7.5 × 10-11 m
D)2.9 × 10-11 m
E)5.1 × 10-10 m
A)1.9 × 10-11 m
B)3.7 × 10-10 m
C)7.5 × 10-11 m
D)2.9 × 10-11 m
E)5.1 × 10-10 m

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
A diatomic molecule has 2.6 × 10-5 eV of rotational energy in the L = 2 quantum state. What is the rotational energy in the L = 1 quantum state?
A)3.4 × 10-6 eV
B)4.1 × 10-6 eV
C)5.3 × 10-6 eV
D)7.8 × 10-6 eV
E)8.7 × 10-6 eV
A)3.4 × 10-6 eV
B)4.1 × 10-6 eV
C)5.3 × 10-6 eV
D)7.8 × 10-6 eV
E)8.7 × 10-6 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
The spacing of the atoms in the H2 molecule is 7.4 x 10-11 m. What is the energy of the L = 1 rotational level?
A)9.0 x 10-2 eV
B)7.0 x 10-2 eV
C)4.5 x 10-2 eV
D)3.0 x 10-2 eV
E)1.5 x 10-2 eV
A)9.0 x 10-2 eV
B)7.0 x 10-2 eV
C)4.5 x 10-2 eV
D)3.0 x 10-2 eV
E)1.5 x 10-2 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Estimate the rotational energy (in eV) for a diatomic hydrogen molecule in the L = 2 quantum state. (The equilibrium separation for the H2 molecule is 0.075 nm.)
A)0.011 eV
B)0.022 eV
C)0.033 eV
D)0.044 eV
E)0.055 eV
A)0.011 eV
B)0.022 eV
C)0.033 eV
D)0.044 eV
E)0.055 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
An unfilled electron state in the valence band is called
A)a hole.
B)an empty electron.
C)a conduction electron.
D)a positron.
E)an empty positron.
A)a hole.
B)an empty electron.
C)a conduction electron.
D)a positron.
E)an empty positron.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
A diatomic molecule has 18 × 10-5 eV of rotational energy in the L = 2 quantum state. What is the rotational energy in the L = 0 quantum state?
A)9.0 × 10-5 eV
B)6.0 × 10-5 eV
C)3.0 × 10-5 eV
D)1.5 × 10-5 eV
E)0 eV
A)9.0 × 10-5 eV
B)6.0 × 10-5 eV
C)3.0 × 10-5 eV
D)1.5 × 10-5 eV
E)0 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
An n-type semiconductor is produced by
A)doping the host crystal with donor impurities.
B)doping the host crystal with accepter impurities.
C)pure crystals of germanium.
D)all of the above.
E)none of the above.
A)doping the host crystal with donor impurities.
B)doping the host crystal with accepter impurities.
C)pure crystals of germanium.
D)all of the above.
E)none of the above.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
Arsenic-doped silicon is
A)a material with holes as the dominant charge carrier.
B)an n-type semiconductor.
C)a p-type semiconductor.
D)all of the above.
E)none of the above.
A)a material with holes as the dominant charge carrier.
B)an n-type semiconductor.
C)a p-type semiconductor.
D)all of the above.
E)none of the above.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
Suppose the first excited vibrational state of a molecule is at 1.50 eV. If the potential is close to that of a simple harmonic oscillator, the energy of the ground state is
A)0 eV
B)0.25 eV
C)0.30 eV
D)0.50 eV
E)0.75 eV
A)0 eV
B)0.25 eV
C)0.30 eV
D)0.50 eV
E)0.75 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
The conductivity of a semiconductor sharply increases when 400 nm (or shorter) wavelength light is shone upon it. This is consistent with what energy gap for this material?
A)5.2 eV
B)3.6 eV
C)3.1 eV
D)1.0 eV
E)0.45 eV
A)5.2 eV
B)3.6 eV
C)3.1 eV
D)1.0 eV
E)0.45 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
What is the occupancy probability at an energy of 12.00 eV for a material with EF = 11.63 eV at a temperature of 500 K?
A)3 x 10-4 %
B)2 x 10-2 %
C)2%
D)6%
E)12%
A)3 x 10-4 %
B)2 x 10-2 %
C)2%
D)6%
E)12%

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
A metal has a Fermi level of 5.50 eV. At 1200 K, what energy will have a 90% occupancy probability?
A)5.79 eV
B)5.73 eV
C)5.56 eV
D)5.44 eV
E)5.27 eV
A)5.79 eV
B)5.73 eV
C)5.56 eV
D)5.44 eV
E)5.27 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
If a metal had a Fermi level of 5.0 eV, what would be the average energy of the electrons at 0 K?
A)8.0 eV
B)6.0 eV
C)3.0 eV
D)0
E)5.0 eV
A)8.0 eV
B)6.0 eV
C)3.0 eV
D)0
E)5.0 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
The energy gap between the valence and conduction bands in a certain semiconductor is 1.25 eV. What is the threshold wavelength for optical absorption in this substance?
A)599 nm
B)639 nm
C)959 nm
D)873 nm
E)995 nm
A)599 nm
B)639 nm
C)959 nm
D)873 nm
E)995 nm

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
If one metal has double the number of conduction electrons per unit volume of a second metal, then its Fermi level is how many times that of the second metal?
A)1.41
B)2.83
C)2.00
D)3.22
E)1.59
A)1.41
B)2.83
C)2.00
D)3.22
E)1.59

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
The conductivity of a semiconductor sharply increases when 400 nm (or shorter) wavelength light is shone upon it. This is consistent with what energy gap for this material?
A)3.6 eV
B)0.45 eV
C)2.0 eV
D)3.1 eV
E)1.0 eV
A)3.6 eV
B)0.45 eV
C)2.0 eV
D)3.1 eV
E)1.0 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
What is the occupancy probability at an energy 1.0% above a Fermi level of 7.0 eV at 300 K?
A)0.06%
B)1.0%
C)3.2%
D)6.3%
E)12%
A)0.06%
B)1.0%
C)3.2%
D)6.3%
E)12%

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
Approximately how many states in the range from 5.0 eV to 5.2 eV are there in a copper bar of volume 5.3 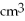 ?
?
A)5.1 x 1022
B)3.2 x 1022
C)1.6 x 1022
D)8.2 x 1021
E)3.2 x 1021
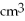 ?
?A)5.1 x 1022
B)3.2 x 1022
C)1.6 x 1022
D)8.2 x 1021
E)3.2 x 1021

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
At room temperature (~295 K with kT ~ 0.025 eV), what fraction of the free electrons in a semiconductor with an energy gap of 1.5 eV can be expected to be in the conduction band?
A)10-9
B)10-13
C)10-30
D)0.03
E)10-15
A)10-9
B)10-13
C)10-30
D)0.03
E)10-15

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
A light emitting diode emits light of approximate wavelength 670 nm. What is the band gap?
A)1.86 eV
B)4.14 eV
C)1.80 eV
D)3.72 eV
E)2.70 eV
A)1.86 eV
B)4.14 eV
C)1.80 eV
D)3.72 eV
E)2.70 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
Silver has a Fermi level of 5.5 eV. At 0 K, what is the speed of the electrons with the Fermi speed?
A)1.4 × 106 m/s
B)1100 m/s
C)2200 m/s
D)0
E)1.1 × 106 m/s
A)1.4 × 106 m/s
B)1100 m/s
C)2200 m/s
D)0
E)1.1 × 106 m/s

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
The Fermi level of a metal is 5.5 eV. What is the number of conduction electrons per unit volume for this metal?
A)5.8 × 1028/m3
B)6.2 × 1028/ m3
C)4.1 × 1028/ m3
D)8.4 × 1028/ m3
E)N/V does not depend on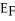
A)5.8 × 1028/m3
B)6.2 × 1028/ m3
C)4.1 × 1028/ m3
D)8.4 × 1028/ m3
E)N/V does not depend on
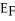

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
A semiconductor has an energy gap of 2.5 eV. What is the longest wavelength light of those listed below that will promote valence electrons into the conduction band?
A)450 nm
B)525 nm
C)600 nm
D)725 nm
E)350 nm
A)450 nm
B)525 nm
C)600 nm
D)725 nm
E)350 nm

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
A material has an energy gap of 2.5 eV. What energy photons in the visible range (1.8 eV to 3.6 eV) would excite electrons into the conduction band?
A)1.8 eV
B)2.5 eV
C)1.8 eV up to 2.5 eV
D)2.5 eV up to 3.6 eV
E)1.5 eV
A)1.8 eV
B)2.5 eV
C)1.8 eV up to 2.5 eV
D)2.5 eV up to 3.6 eV
E)1.5 eV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
If the voltage gain for a transistor amplifier is 60 in a circuit with an output resistance of 10,000 ohms, what is the peak output current if the peak input voltage is 0.05 V?
A)3 x 10-4 A
B)4 x 10-4 A
C)5 x 10-4 A
D)6 x 10-4 A
E)60 μA
A)3 x 10-4 A
B)4 x 10-4 A
C)5 x 10-4 A
D)6 x 10-4 A
E)60 μA

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


