Exam 40: Molecules and Solids
Exam 1: Introduction, Measurement, Estimating71 Questions
Exam 2: Describing Motion: Kinematics in One Dimension119 Questions
Exam 3: Kinematics in Two or Three Dimensions; Vectors100 Questions
Exam 4: Dynamics: Newtons Laws of Motion86 Questions
Exam 5: Using Newtons Laws: Friction, Circular Motion, Drag Forces68 Questions
Exam 6: Gravitation and Newtons6 Synthesis64 Questions
Exam 7: Work and Energy69 Questions
Exam 8: Conservation of Energy95 Questions
Exam 9: Linear Momentum85 Questions
Exam 10: Rotational Motion99 Questions
Exam 11: Angular Momentum; General Rotation45 Questions
Exam 12: Static Equilibrium; Elasticity and Fracture61 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations102 Questions
Exam 15: Wave Motion74 Questions
Exam 16: Sound75 Questions
Exam 17: Temperature, Thermal Expansion, and the Ideal Gas Law83 Questions
Exam 18: Kinetic Theory of Gases37 Questions
Exam 19: Heat and the First Law of Thermodynamics96 Questions
Exam 20: Second Law of Thermodynamics77 Questions
Exam 21: Electric Charge and Electric Field97 Questions
Exam 22: Gausss Law44 Questions
Exam 23: Electric Potential70 Questions
Exam 24: Capacitance, Dielectrics, Electric Energy Storage73 Questions
Exam 25: Electric Currents and Resistance71 Questions
Exam 26: Dc Circuits110 Questions
Exam 27: Magnetism102 Questions
Exam 28: Sources of Magnetic Field63 Questions
Exam 29: Electromagnetic Induction and Faradays Law116 Questions
Exam 30: Inductance, Electromagnetic Oscillations, and Ac Circuits108 Questions
Exam 31: Maxwells Equations and Electromagnetic Waves76 Questions
Exam 32: Light: Reflection and Refraction118 Questions
Exam 33: Lenses and Optical Instruments134 Questions
Exam 34: The Wave Nature of Light; Interference77 Questions
Exam 35: Diffraction and Polarization68 Questions
Exam 36: Special Theory of Relativity69 Questions
Exam 37: Early Quantum Theory and Models of the Atom95 Questions
Exam 38: Quantum Mechanics42 Questions
Exam 39: Quantum Mechanics of Atoms62 Questions
Exam 40: Molecules and Solids56 Questions
Exam 41: Nuclear Physics and Radioactivity82 Questions
Exam 42: Nuclear Energy: Efects and Uses of Radiation69 Questions
Exam 43: Elementary Particle66 Questions
Exam 44: Astrophysics and Cosmology36 Questions
Select questions type
A diatomic quantum mechanical vibrator in its ground state has energy E. This same vibrator in its third state has energy
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
C
Approximately how many states in the range from 5.0 eV to 5.2 eV are there in a copper bar of volume 5.3 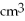 ?
?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
The conductivity of a semiconductor sharply increases when 400 nm (or shorter) wavelength light is shone upon it. This is consistent with what energy gap for this material?
(Multiple Choice)
4.9/5  (30)
(30)
The spacing of the atoms in the H2 molecule is 7.4 x 10-11 m. What is the energy of the L = 1 rotational level?
(Multiple Choice)
5.0/5  (41)
(41)
If one metal has double the number of conduction electrons per unit volume of a second metal, then its Fermi level is how many times that of the second metal?
(Multiple Choice)
4.8/5  (33)
(33)
The Fermi level of a metal is 5.5 eV. What is the number of conduction electrons per unit volume for this metal?
(Multiple Choice)
4.8/5  (43)
(43)
In a good insulator, the highest energy band containing electrons, called the valence band, is
(Multiple Choice)
4.9/5  (34)
(34)
A metal has a Fermi level of 5.50 eV. At 1200 K, what energy will have a 90% occupancy probability?
(Multiple Choice)
4.8/5  (41)
(41)
The potential energy for the formation of a hydrogen molecule can be approximated by U = -A + BR + C/R, where R is the nuclear separation. Given that A = -78.5 eV, B = 2.57 × 1011 eV/m , and C = 1.44 × 10-9 eV∙m. At what nuclear separation is the potential energy a minimum?
(Multiple Choice)
4.9/5  (42)
(42)
A diatomic molecule has 2.6 × 10-5 eV of rotational energy in the L = 2 quantum state. What is the rotational energy in the L = 1 quantum state?
(Multiple Choice)
4.7/5  (39)
(39)
A semiconductor has an energy gap of 2.5 eV. What is the longest wavelength light of those listed below that will promote valence electrons into the conduction band?
(Multiple Choice)
4.7/5  (28)
(28)
If the voltage gain for a transistor amplifier is 60 in a circuit with an output resistance of 10,000 ohms, what is the peak output current if the peak input voltage is 0.05 V?
(Multiple Choice)
4.8/5  (26)
(26)
A diatomic molecule has 18 × 10-5 eV of rotational energy in the L = 2 quantum state. What is the rotational energy in the L = 0 quantum state?
(Multiple Choice)
4.8/5  (36)
(36)
If a battery is connected to a diode with the positive terminal to the p side and the negative terminal to the n side, then diode is said to be
(Multiple Choice)
4.7/5  (28)
(28)
What is the occupancy probability at an energy 1.0% above a Fermi level of 7.0 eV at 300 K?
(Multiple Choice)
4.9/5  (36)
(36)
What is the occupancy probability at an energy of 12.00 eV for a material with EF = 11.63 eV at a temperature of 500 K?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)