Deck 7: Substitution Reactions: the Sn2 and Sn1 Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/55
Play
Full screen (f)
Deck 7: Substitution Reactions: the Sn2 and Sn1 Reactions
1
Which of the following expressions correctly states the rate law for the SN2 reaction shown here? (NaN3= sodium azide)
![<strong>Which of the following expressions correctly states the rate law for the S<sub>N</sub>2 reaction shown here? (NaN<sub>3</sub>= sodium azide) </strong> A) rate =k[2 -bromopropane ] B) rate =k [sodium azide] C) rate =k[2 -bromopropane ] [sodium azide] D) rate =k[2 -bromopropane }2[ sodium azide ] E) It is impossible to tell from the information given.](https://storage.examlex.com/TB34225555/11ec81bf_f161_1212_9b32_c72a882dbc73_TB34225555_11.jpg)
A) rate =k[2 -bromopropane ]
B) rate =k [sodium azide]
C) rate =k[2 -bromopropane ] [sodium azide]
D) rate =k[2 -bromopropane }2[ sodium azide ]
E) It is impossible to tell from the information given.
![<strong>Which of the following expressions correctly states the rate law for the S<sub>N</sub>2 reaction shown here? (NaN<sub>3</sub>= sodium azide) </strong> A) rate =k[2 -bromopropane ] B) rate =k [sodium azide] C) rate =k[2 -bromopropane ] [sodium azide] D) rate =k[2 -bromopropane }2[ sodium azide ] E) It is impossible to tell from the information given.](https://storage.examlex.com/TB34225555/11ec81bf_f161_1212_9b32_c72a882dbc73_TB34225555_11.jpg)
A) rate =k[2 -bromopropane ]
B) rate =k [sodium azide]
C) rate =k[2 -bromopropane ] [sodium azide]
D) rate =k[2 -bromopropane }2[ sodium azide ]
E) It is impossible to tell from the information given.
rate =k[2 -bromopropane ] [sodium azide]
2
Which of the following is a likely product of the reaction sequence shown?
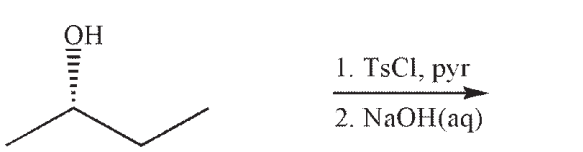
A)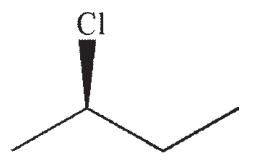
B)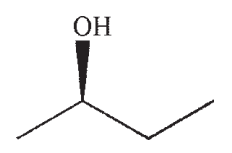
C)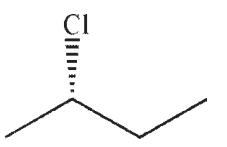
D)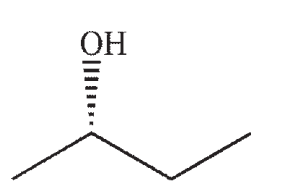
E)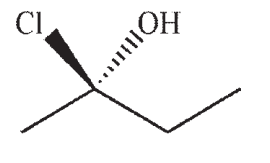
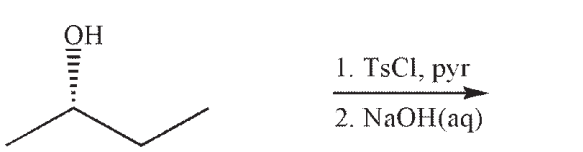
A)

B)

C)

D)

E)


3
Chemicals such as dimethyl sulfate and iodomethane are considered biologically hazardous because:
A)They can disrupt the body's endocrine system.
B)They are acute respiratory hazards, leading to pulmonary edema.
C)They can alkylate nucleotide bases, leading to mutagenic and carcinogenic effects.
D)They can cause damage to the liver and kidneys.
E)They are corrosive to the eyes and upper respiratory tract.
A)They can disrupt the body's endocrine system.
B)They are acute respiratory hazards, leading to pulmonary edema.
C)They can alkylate nucleotide bases, leading to mutagenic and carcinogenic effects.
D)They can cause damage to the liver and kidneys.
E)They are corrosive to the eyes and upper respiratory tract.
They can alkylate nucleotide bases, leading to mutagenic and carcinogenic effects.
4
In which of the following instances would it be expected that the reaction would always have an equilibrium constant that is greater than 1 ?
A) ΔH and ΔS for the reaction are both positive.
B) Δ H for the reaction is positive, while Δ S for the reaction is negative.
C) ΔH and ΔS for the reaction are both negative.
D) Δ H and ΔS for the reaction are both zero.
E) ΔH for the reaction is negative, while Δ S for the reaction is positive.
A) ΔH and ΔS for the reaction are both positive.
B) Δ H for the reaction is positive, while Δ S for the reaction is negative.
C) ΔH and ΔS for the reaction are both negative.
D) Δ H and ΔS for the reaction are both zero.
E) ΔH for the reaction is negative, while Δ S for the reaction is positive.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a Lewis acid but not a Brønsted acid?
A) NH3
B) H2O
C) AlCl3
D) CH3OH
E) CH3CH2SH
A) NH3
B) H2O
C) AlCl3
D) CH3OH
E) CH3CH2SH

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
6
Which of these carbocations is most stable? 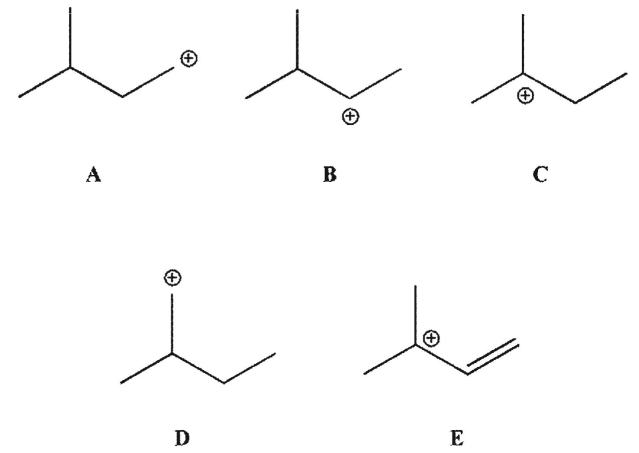
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the product of the following reaction. 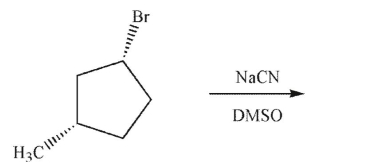
A)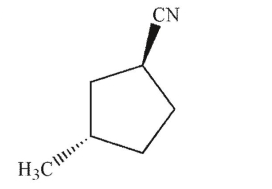
B)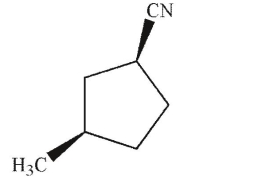
C)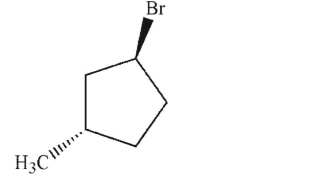
D)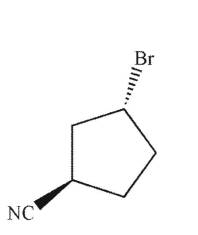
E)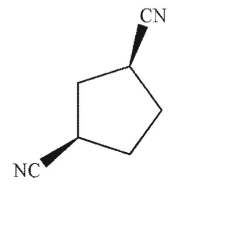

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the product of this reaction?
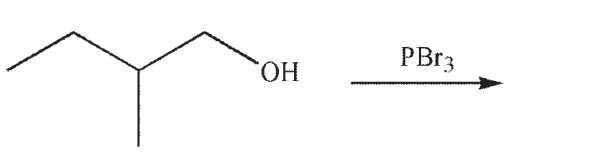
A)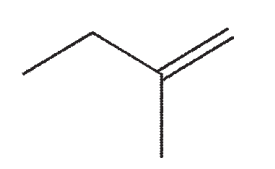
B)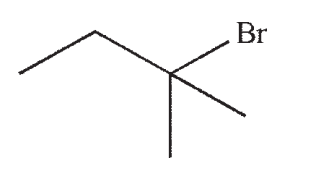
C)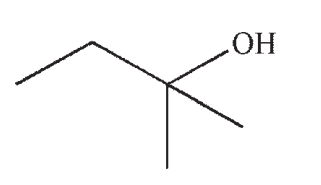
D)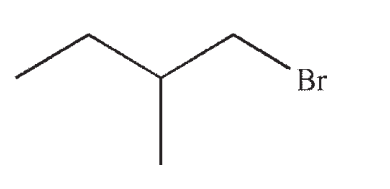
E)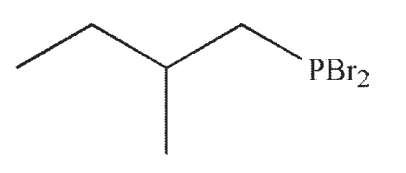

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following would react most rapidly in an SN1 reaction?
A)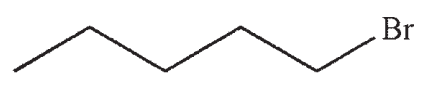
B)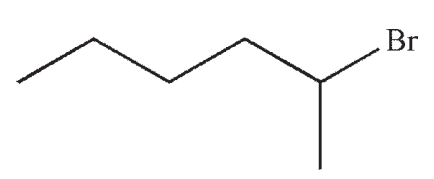
C)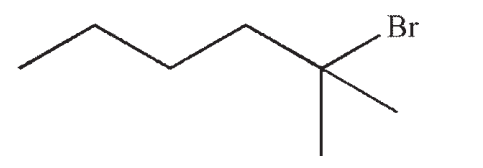
D)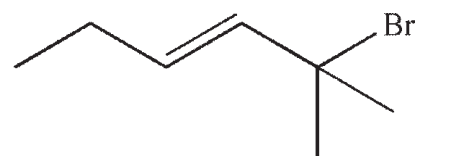
E)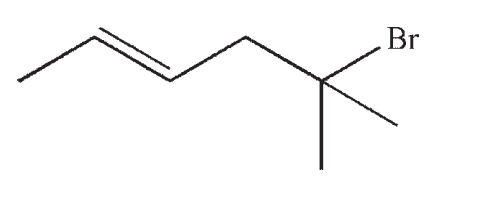
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these choices is the best leaving group?
A) OH-
B) NH3
C) Cl-
D) CH3O-
E) H2O
A) OH-
B) NH3
C) Cl-
D) CH3O-
E) H2O

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following would not be a valid reason for a substitution reaction being irreversible?
A)The reaction is highly exothermic.
B)The reaction requires a catalyst to proceed.
C)One product is a gas that bubbles out of the reaction medium.
D)One product crystallizes out of the reaction medium.
E)One reactant is present in a very large quantity relative to the other.
A)The reaction is highly exothermic.
B)The reaction requires a catalyst to proceed.
C)One product is a gas that bubbles out of the reaction medium.
D)One product crystallizes out of the reaction medium.
E)One reactant is present in a very large quantity relative to the other.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
12
For the reaction profile shown below, which statement(s) could be made? 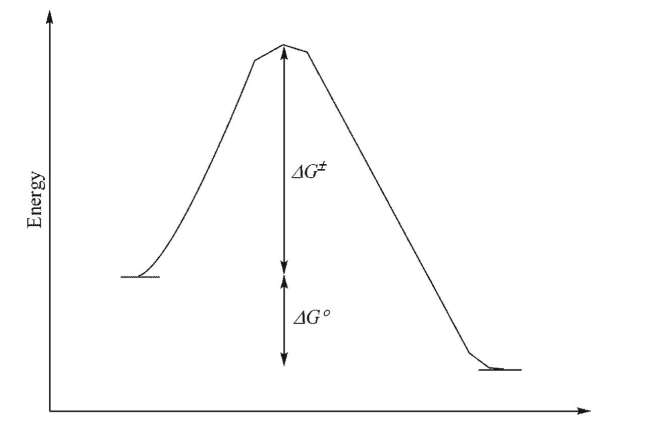
I.The transition state will look more like the starting materials than the products.
II.The reaction is endothermic.
III.The equilibrium constant for the forward reaction will be larger than 1.
IV.The activation barriers for the forward and reverse reactions are equal.
A)I only
B)I and II
C)I and III
D)I, II and III
E)I, III and IV

I.The transition state will look more like the starting materials than the products.
II.The reaction is endothermic.
III.The equilibrium constant for the forward reaction will be larger than 1.
IV.The activation barriers for the forward and reverse reactions are equal.
A)I only
B)I and II
C)I and III
D)I, II and III
E)I, III and IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
13
For the following reaction, what will be the effect on the rate if [NaN3] is tripled?
![<strong>For the following reaction, what will be the effect on the rate if [NaN<sub>3</sub>] is tripled? </strong> A) The rate doubles. B) The rate triples. C) The rate quadruples. D) The rate stays the same. E) There is not enough information to determine the effect.](https://storage.examlex.com/TB34225555/11ec81c4_dea2_8a82_9b32_91842c356645_TB34225555_11.jpg)
A) The rate doubles.
B) The rate triples.
C) The rate quadruples.
D) The rate stays the same.
E) There is not enough information to determine the effect.
![<strong>For the following reaction, what will be the effect on the rate if [NaN<sub>3</sub>] is tripled? </strong> A) The rate doubles. B) The rate triples. C) The rate quadruples. D) The rate stays the same. E) There is not enough information to determine the effect.](https://storage.examlex.com/TB34225555/11ec81c4_dea2_8a82_9b32_91842c356645_TB34225555_11.jpg)
A) The rate doubles.
B) The rate triples.
C) The rate quadruples.
D) The rate stays the same.
E) There is not enough information to determine the effect.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following factors would not be expected to affect the entropy of a reaction?
A)Acyclic reactants are transformed into cyclic products.
B)A weak bond in the reactant is replaced with a strong bond in the product.
C)Two molecules of reactants are transformed into three molecules of products.
D)A double bond in the reactant is converted into a single bond in the product.
E)Gaseous reactants are transformed into solid products.
A)Acyclic reactants are transformed into cyclic products.
B)A weak bond in the reactant is replaced with a strong bond in the product.
C)Two molecules of reactants are transformed into three molecules of products.
D)A double bond in the reactant is converted into a single bond in the product.
E)Gaseous reactants are transformed into solid products.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the HOMO and the LUMO in the following SN2 reaction.

A) The HOMO is a bonding C-Br σ and the LUMO is an antibonding C-Br σ* .
B) The HOMO is a bonding C-Br σ* and the LUMO is an antibonding C-Br σ .
C) The HOMO is a nonbonding (n) on hydroxide ion and the LUMO is an antibonding OH σ*.
D) The HOMO is a nonbonding (n) on hydroxide ion and the LUMO is an antibonding C-Br σ* .
E) None of these choices correctly identify the HOMO and LUMO in this reaction.

A) The HOMO is a bonding C-Br σ and the LUMO is an antibonding C-Br σ* .
B) The HOMO is a bonding C-Br σ* and the LUMO is an antibonding C-Br σ .
C) The HOMO is a nonbonding (n) on hydroxide ion and the LUMO is an antibonding OH σ*.
D) The HOMO is a nonbonding (n) on hydroxide ion and the LUMO is an antibonding C-Br σ* .
E) None of these choices correctly identify the HOMO and LUMO in this reaction.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following electrophiles would undergo an SN2 reaction with thiolate ion (HS-) at the fastest rate?
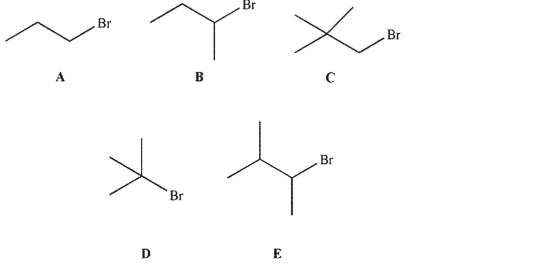
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following ideas best explains why SN2 reactions proceed with inversion of configuration?
A) steric hindrance
B) orbital overlap
C) nucleophile strength
D) solvent effects
E) leaving group ability
A) steric hindrance
B) orbital overlap
C) nucleophile strength
D) solvent effects
E) leaving group ability

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following will react most slowly with cyanide nucleophile (NC-) in an SN2 reaction?
A)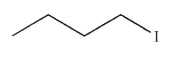
B)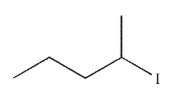
C)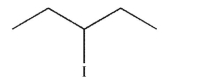
D)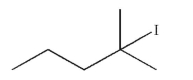
E) CH3I
A)

B)

C)

D)

E) CH3I

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements about Lewis acids and bases is false?
A)Lewis acids are also known as electrophiles.
B)A vacant orbital of a Lewis acid will interact with a filled orbital of a Lewis base to form a sigma bond.
C)All Brønsted bases are also Lewis bases.
D)Typically the LUMO of the Lewis base interacts with the HOMO of the Lewis acid.
E)The reaction of hydride anion with a methyl cation would be an example of a Lewis base/Lewis acid reaction.
A)Lewis acids are also known as electrophiles.
B)A vacant orbital of a Lewis acid will interact with a filled orbital of a Lewis base to form a sigma bond.
C)All Brønsted bases are also Lewis bases.
D)Typically the LUMO of the Lewis base interacts with the HOMO of the Lewis acid.
E)The reaction of hydride anion with a methyl cation would be an example of a Lewis base/Lewis acid reaction.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the rate limiting step in an SN1 reaction?
A)
B)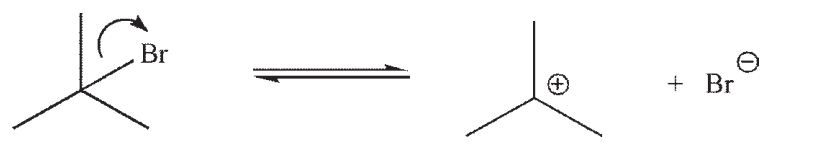
C)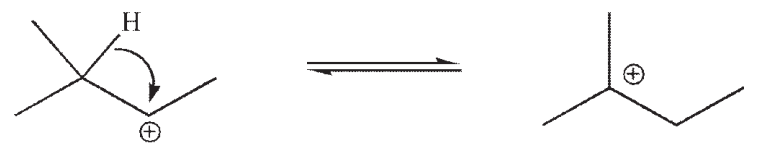
D)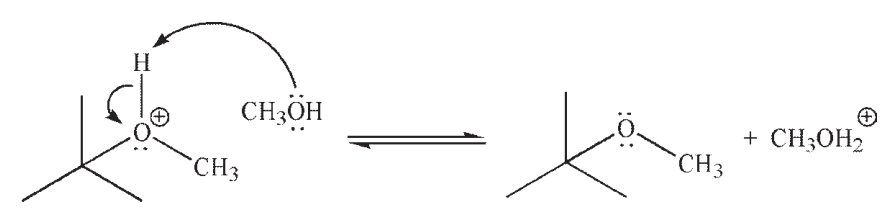
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
21
Identify the Lewis acid and the Lewis base in the reaction shown here.
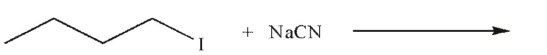

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
22
Hydroxide is a poor leaving group. Describe three ways to convert the hydroxyl group to a better leaving group.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
23
Draw the product of the following sequence of reactions. Show stereochemistry at any stereogenic carbon.
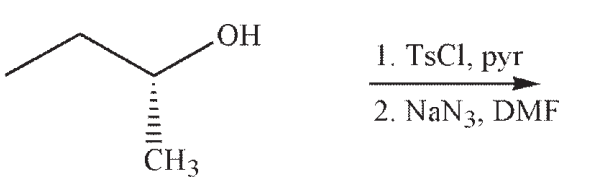
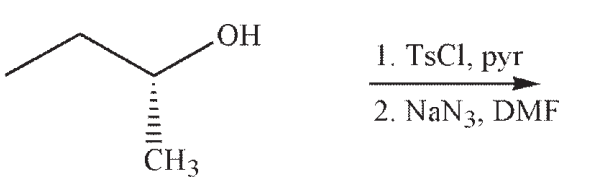

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these is the most efficient method for making the ether shown in the highest possible yield using a Williamson ether synthesis?
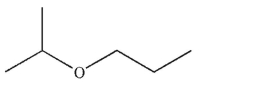
A)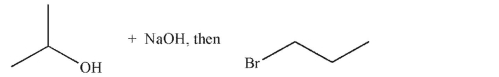
B)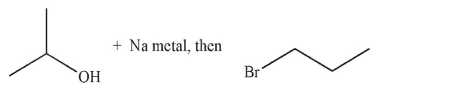
C)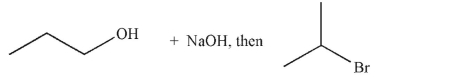
D)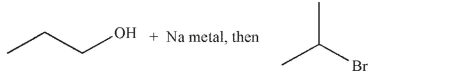
E) Methods b and d are equally efficient.

A)

B)
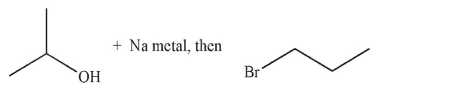
C)
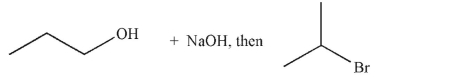
D)
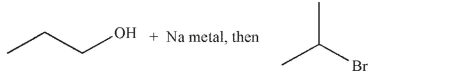
E) Methods b and d are equally efficient.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the HOMO and the LUMO in the reaction shown here.
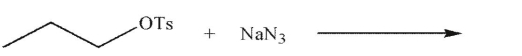
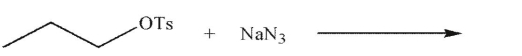

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
26
Draw an energy diagram for the SN2 reaction shown here. Show the correct relative energies of the starting materials and products.
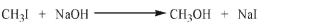
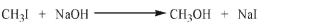

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
27
Draw a mechanism for the following transformation. Show all curved arrows, necessary lone pairs, and nonzero formal charges.
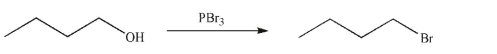


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
28
Explain why SN2 reactions proceed with inversion of configuration at a stereogenic carbon, but product mixtures in SN1 reactions show both inversion and retention of configuration.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
29
What is the most likely stereochemical outcome of the SN1 reaction shown here?
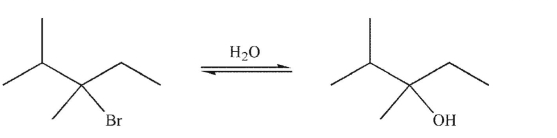
A) complete inversion of configuration
B) complete retention of configuration
C) racemization
D) more retention than inversion
E) more inversion than retention

A) complete inversion of configuration
B) complete retention of configuration
C) racemization
D) more retention than inversion
E) more inversion than retention

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
30
SN1 reactions run in polar solvents have faster rates than SN1 reactions run in nonpolar solvents.
Which statement provides the best explanation for the difference in rates?
A) Polar solvents stabilize the transition state in the rate-determining step.
B) Nonpolar solvents stabilize the transition state in the rate-determining step.
C) Polar solvents stabilize the carbocation intermediate.
D) Nonpolar solvents stabilize the carbocation intermediate.
E) The reaction mechanism is different in polar solvents than in nonpolar solvents.
Which statement provides the best explanation for the difference in rates?
A) Polar solvents stabilize the transition state in the rate-determining step.
B) Nonpolar solvents stabilize the transition state in the rate-determining step.
C) Polar solvents stabilize the carbocation intermediate.
D) Nonpolar solvents stabilize the carbocation intermediate.
E) The reaction mechanism is different in polar solvents than in nonpolar solvents.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
31
What sequence of reactions could be used for the following synthesis?
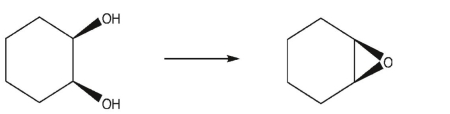
A)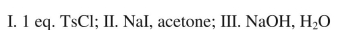
B)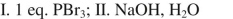
C)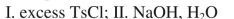
D)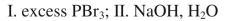
E)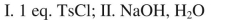

A)
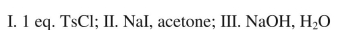
B)
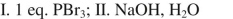
C)
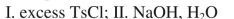
D)
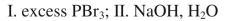
E)
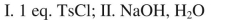

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
32
Use molecular orbital interactions to illustrate why SN2 reactions proceed with inversion of configuration at the substrate.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds is the product of the reaction shown that displays retention of configuration compared to the starting material? 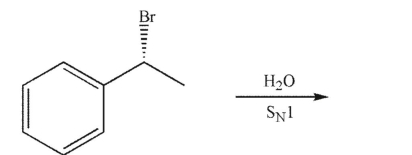
A)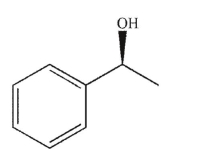
B)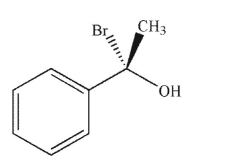
C)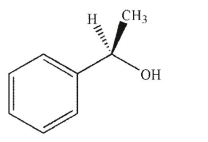
D)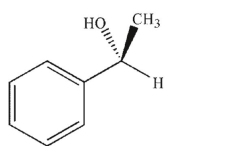
E)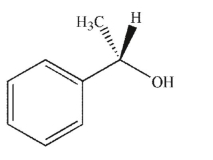

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
34
Draw the SN2 product of the following reaction. Show stereochemistry at any stereogenic carbon.
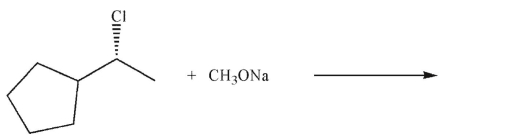
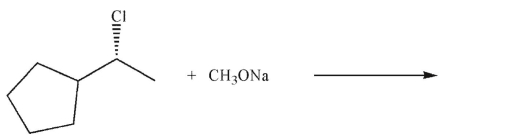

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the weakest nucleophile in protic solvent in an SN2 reaction, and why?
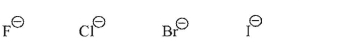
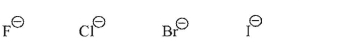

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
36
Draw the expected transition state geometry for each of these SN2 reactions based on the reaction enthalpy values. (Hint: Will the TS look more like the reactant or the product?):
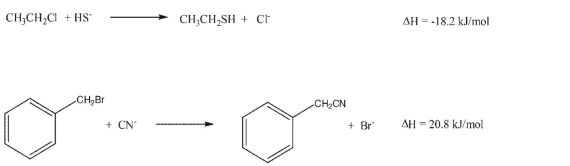


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
37
Which structure is the most likely SN1 product under the following conditions?
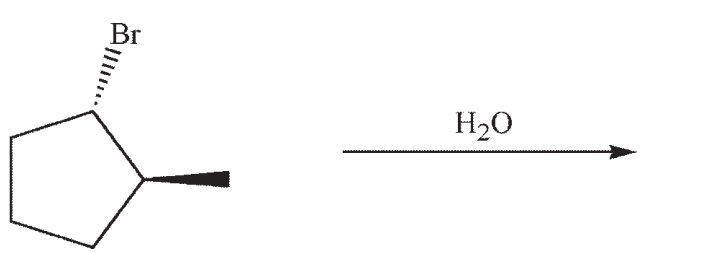
A)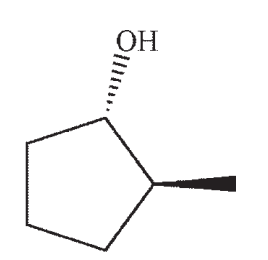
B)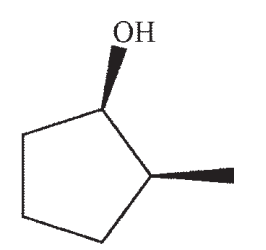
C)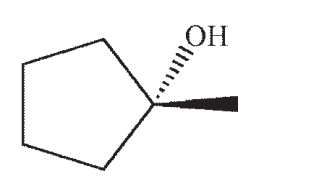
D)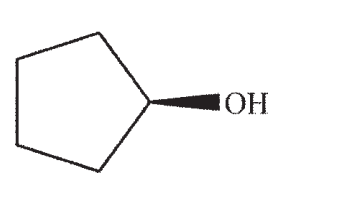
E) None of these products will form.

A)

B)

C)

D)

E) None of these products will form.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
38
Which of these alkyl bromides will react faster in an SN2 reaction? Explain.
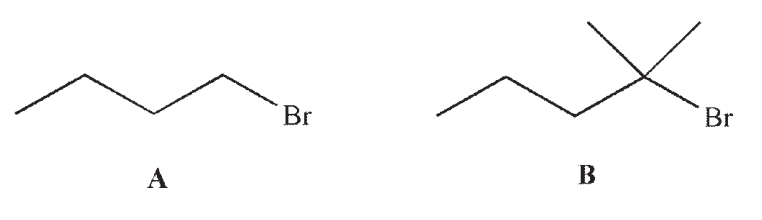
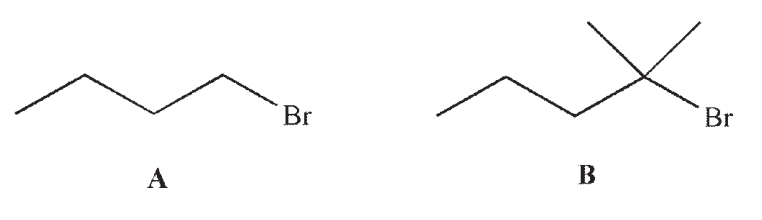

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
39
What is the likely product of reaction of dimethylamine with excess methyl iodide?
A) HNI2
B)HNMe3+I-
C)NMe4+I-
D) HNMeI
E) Me3N
A) HNI2
B)HNMe3+I-
C)NMe4+I-
D) HNMeI
E) Me3N

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
40
In each pair shown, which SN2 reaction would proceed faster?
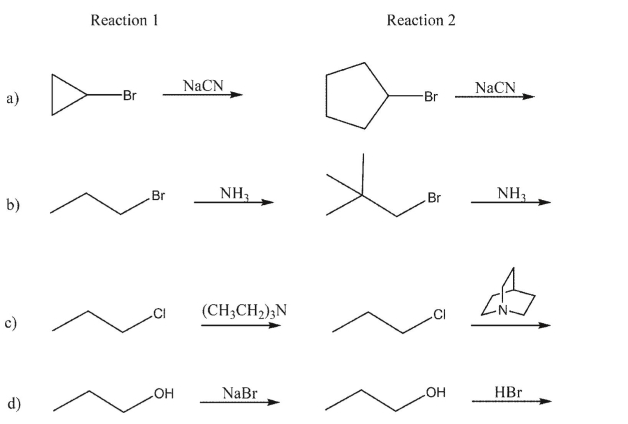


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
41
Explain why SN1 reactions are favored by the use of a polar solvent.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
42
The final step in the SN1 reaction of water with tert-butyl bromide to form tert-butyl alcohol is the deprotonation of the oxonium ion to give the alcohol plus hydronium:

The relative energies of the starting materials and products in this step are expected to be very similar. Explain why in practice this reaction proceeds essentially to completion.

The relative energies of the starting materials and products in this step are expected to be very similar. Explain why in practice this reaction proceeds essentially to completion.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
43
Outline a multistep synthesis of the target molecule from the starting material shown.You may
use any organic or inorganic reagents.Show the reagents necessary for each step and the product
of each step.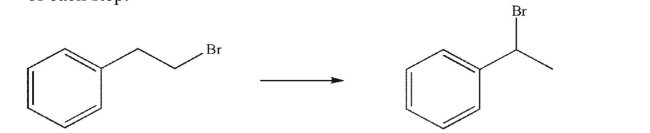
use any organic or inorganic reagents.Show the reagents necessary for each step and the product
of each step.


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
44
Predict the product of the following reaction. 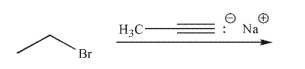


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
45
Outline a multistep synthesis of the target molecule from the starting material shown.You may
use any organic or inorganic reagents.Show the reagents necessary for each step and the product
of each step.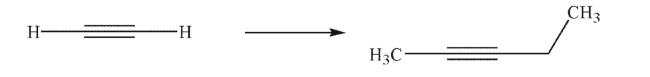
use any organic or inorganic reagents.Show the reagents necessary for each step and the product
of each step.


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
46
Outline a multistep synthesis of the target molecule from the starting material shown.You may
use any organic or inorganic reagents.Show the reagents necessary for each step and the product
of each step.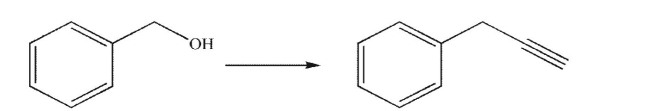
use any organic or inorganic reagents.Show the reagents necessary for each step and the product
of each step.


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
47
Which of these molecules will react faster in an SN1 reaction with methanol? Explain.
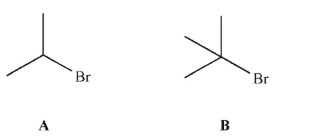


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
48
Show how you would perform the following synthesis.Show the reagents you would use for each step and the organic intermediate formed in each step. 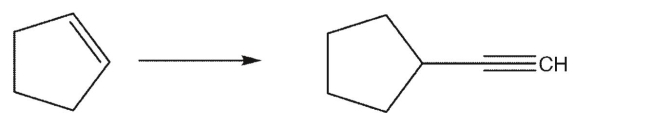


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
49
An intramolecular Williamson ether synthesis may be used to produce epoxides.Predict the
product of the following reaction conditions and provide a mechanism for the transformation.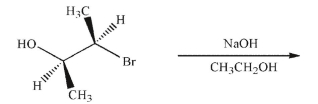
product of the following reaction conditions and provide a mechanism for the transformation.


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the structure of the alkyl bromide starting material necessary to react with a cyanide ion to
produce the molecule shown.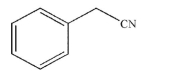
produce the molecule shown.


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
51
Which of these carbocations is more stable? Explain.
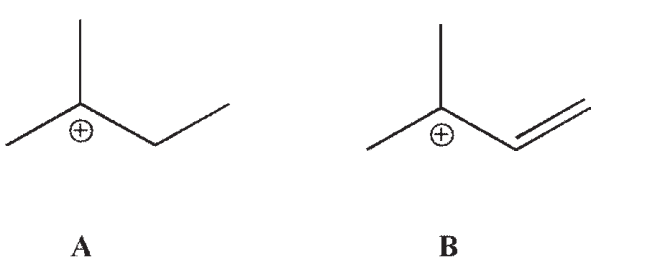


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
52
Provide the missing reagents in the following reaction sequence.
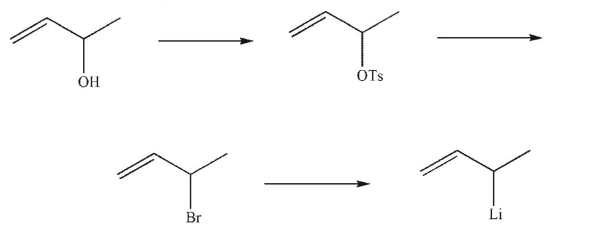


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
53
Based on nucleophile, substrate, and reaction conditions, state the most likely reaction mechanism (SN1 or SN2) in each example. Draw the products, including stereochemistry as needed.
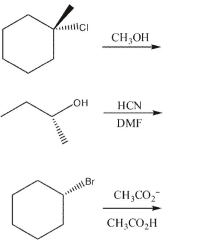


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
54
Draw an energy diagram for the SN1 reaction between tert-butyl bromide and water; assume that the reaction is overall exothermic.



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
55
Supply the reagents/intermediates in the multistep synthesis shown below.
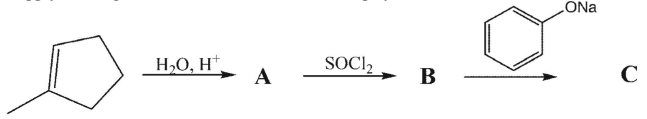
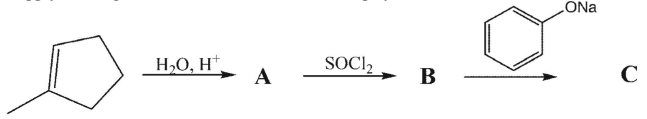

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck


