Exam 7: Substitution Reactions: the Sn2 and Sn1 Reactions
Exam 1: Atoms and Molecules; Orbitals and Bonding64 Questions
Exam 2: Alkanes65 Questions
Exam 3: Alkenes and Alkynes70 Questions
Exam 4: Stereochemistry68 Questions
Exam 5: Rings60 Questions
Exam 6: Substituted Alkanes: Alkyl Halides, Alcohols, Amines, Ethers,thiols, and Thioethers68 Questions
Exam 7: Substitution Reactions: the Sn2 and Sn1 Reactions55 Questions
Exam 8: Elimination Reactions: the E1 and E2 Reactions45 Questions
Exam 9: Analytical Chemistry: Spectroscopy65 Questions
Exam 10: Electrophilic Additions to Alkenes68 Questions
Exam 11: More Additions to Bonds65 Questions
Exam 12: Radical Reactions65 Questions
Exam 13: Dienes and the Allyl System: 2p Orbitals in Conjugation68 Questions
Exam 14: Aromaticity66 Questions
Exam 15: Substitution Reactions of Aromatic Compounds68 Questions
Exam 16: Carbonyl Chemistry 1: Addition Reactions73 Questions
Exam 17: Carboxylic Acids66 Questions
Exam 18: Derivatives of Carboxylic Acids: Acyl Compounds68 Questions
Exam 19: Carbonyl Chemistry 2: Reactions at the Α Position71 Questions
Exam 20: Special Topic: Carbohydrates40 Questions
Exam 21: Special Topic: Bio-Organic Chemistry40 Questions
Exam 22: Special Topic: Amino Acids and Polyamino Acids Peptides and Proteins39 Questions
Exam 23: Special Topic: Reactions Controlled by Orbital Symmetry46 Questions
Exam 24: Special Topic: Intramolecular Reactions and Neighboring Group Participation40 Questions
Select questions type
Which of the following is the weakest nucleophile in protic solvent in an SN2 reaction, and why?
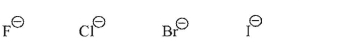
Free
(Essay)
5.0/5  (40)
(40)
Correct Answer:
Fluoride is the weakest nucleophile in protic solvent in an SN2 reaction. Because it is the smallest of the nucleophiles shown, it is most easily solvated and must shed solvent molecules to react with an electrophile.
Which of the following is a likely product of the reaction sequence shown?
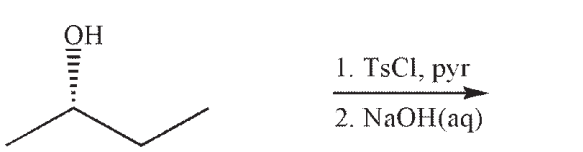
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
B
SN1 reactions run in polar solvents have faster rates than SN1 reactions run in nonpolar solvents.
Which statement provides the best explanation for the difference in rates?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
Supply the reagents/intermediates in the multistep synthesis shown below.
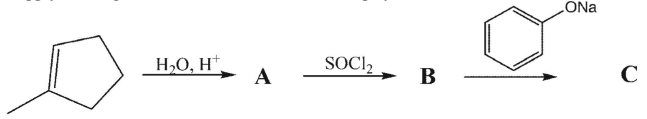
(Essay)
4.8/5  (30)
(30)
Which of the following is the rate limiting step in an SN1 reaction?
(Multiple Choice)
4.9/5  (31)
(31)
Draw an energy diagram for the SN1 reaction between tert-butyl bromide and water; assume that the reaction is overall exothermic.

(Essay)
4.9/5  (38)
(38)
For the following reaction, what will be the effect on the rate if [NaN3] is tripled?
![For the following reaction, what will be the effect on the rate if [NaN<sub>3</sub>] is tripled?](https://storage.examlex.com/TB34225555/11ec81c4_dea2_8a82_9b32_91842c356645_TB34225555_11.jpg)
(Multiple Choice)
4.8/5  (41)
(41)
Draw the structure of the alkyl bromide starting material necessary to react with a cyanide ion to
produce the molecule shown. 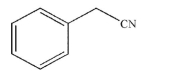
(Essay)
4.9/5  (34)
(34)
Hydroxide is a poor leaving group. Describe three ways to convert the hydroxyl group to a better leaving group.
(Essay)
4.7/5  (27)
(27)
Which of the following would react most rapidly in an SN1 reaction?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is a Lewis acid but not a Brønsted acid?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following would not be a valid reason for a substitution reaction being irreversible?
(Multiple Choice)
4.9/5  (37)
(37)
Based on nucleophile, substrate, and reaction conditions, state the most likely reaction mechanism (SN1 or SN2) in each example. Draw the products, including stereochemistry as needed.
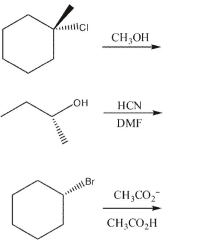
(Essay)
4.9/5  (35)
(35)
Outline a multistep synthesis of the target molecule from the starting material shown.You may
use any organic or inorganic reagents.Show the reagents necessary for each step and the product
of each step. 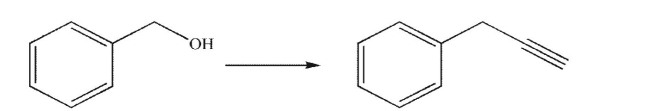
(Essay)
4.8/5  (42)
(42)
Draw an energy diagram for the SN2 reaction shown here. Show the correct relative energies of the starting materials and products.
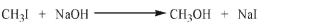
(Essay)
4.9/5  (36)
(36)
Explain why SN2 reactions proceed with inversion of configuration at a stereogenic carbon, but product mixtures in SN1 reactions show both inversion and retention of configuration.
(Essay)
4.9/5  (32)
(32)
Which structure is the most likely SN1 product under the following conditions?
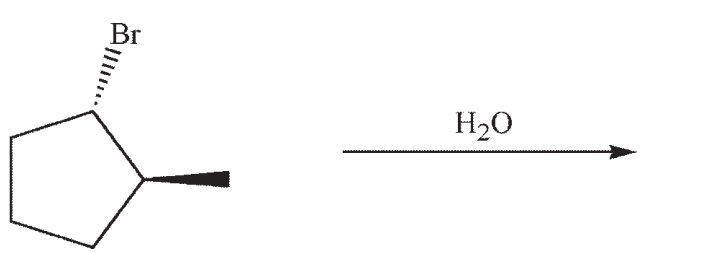
(Multiple Choice)
4.7/5  (39)
(39)
Showing 1 - 20 of 55
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)