Deck 3: Bioenergetics, Enzymes and Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/80
Play
Full screen (f)
Deck 3: Bioenergetics, Enzymes and Metabolism
1
Reactions that lose heat are termed:
A)entropic
B)endothermic
C)exothermic
D)unreactive
A)entropic
B)endothermic
C)exothermic
D)unreactive
C
2
Enthalpy is ________.
A)the energy available to do work
B)the total energy content of a system
C)named after J. Willard Gibbs
D)the energy available to do work and the total energy content of a system
E)All of these are correct.
A)the energy available to do work
B)the total energy content of a system
C)named after J. Willard Gibbs
D)the energy available to do work and the total energy content of a system
E)All of these are correct.
B
3
After allowing the reaction to proceed, you find that the the final concentrations of each once equilibrium is achieved is
Will the reaction proceed in a forward manner, namely with production of increased concentrations of products?
A)yes, because Keq is greater than 1
B)no, because Keq is greater than 1
C)yes, because Keq is less than 1
D)no, because Keq is less than 1
Will the reaction proceed in a forward manner, namely with production of increased concentrations of products?
A)yes, because Keq is greater than 1
B)no, because Keq is greater than 1
C)yes, because Keq is less than 1
D)no, because Keq is less than 1
no, because Keq is less than 1
4
The statement that events in the universe proceed from a state of higher energy to one of lower energy describes the_____________________.
A)first law of thermodynamics
B)second law of thermodynamics
C)third law of thermodynamics
D)fourth law of thermodynamics
A)first law of thermodynamics
B)second law of thermodynamics
C)third law of thermodynamics
D)fourth law of thermodynamics

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
5
Given the equation G = H - T S, which set of conditions would result in a reaction that is unambiguously nonspontaneous?
A)entropy decreases and the reaction is endothermic
B)entropy increases and the reaction is exothermic
C)entropy stays the same and there is no change in enthalpy
D)entropy decreases and the reaction is exothermic
E)entropy increases and the reaction is endothermic
A)entropy decreases and the reaction is endothermic
B)entropy increases and the reaction is exothermic
C)entropy stays the same and there is no change in enthalpy
D)entropy decreases and the reaction is exothermic
E)entropy increases and the reaction is endothermic

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
6
Which reaction below might be a suitable coupled reaction for the reaction
A + B -> C + D ( G = -8.7 kcal/mole)?
A)E + F -> G + H ( G = -5.4 kcal/mole)
B)B + F -> G + H ( G = -5.4 kcal/mole)
C)C + F -> G + H ( G = +8.3 kcal/mole)
D)C + F -> G + H ( G = +9.7 kcal/mole)
E)A + F -> G + H ( G = +10.2 kcal/mole)
A + B -> C + D ( G = -8.7 kcal/mole)?
A)E + F -> G + H ( G = -5.4 kcal/mole)
B)B + F -> G + H ( G = -5.4 kcal/mole)
C)C + F -> G + H ( G = +8.3 kcal/mole)
D)C + F -> G + H ( G = +9.7 kcal/mole)
E)A + F -> G + H ( G = +10.2 kcal/mole)

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
7
After allowing the reaction to proceed, you find that the the final concentrations of each reagent once equilibrium is achieved is
What is the value of the equilibrium constant Keq?
A)18
B)1.8
C)0.18
D).001
What is the value of the equilibrium constant Keq?
A)18
B)1.8
C)0.18
D).001

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
8
You are observing a reaction and discover that the reaction vessel is warm to the touch. The reaction also results in an increase in entropy. Which statement correctly confirms whether or not the reaction is spontaneous and explains why?
A)The reaction is exothermic (- H)as demonstrated by the warm reaction vessel and entropy is increased (+ S). The reaction has a negative G and is, therefore, spontaneous.
B)The reaction is exothermic (+ H)as demonstrated by the warm reaction vessel and entropy is increased (+ S). The reaction therefore has a negative G and is, therefore, spontaneous.
C)The reaction is exothermic (+ H)as demonstrated by the warm reaction vessel and entropy is increased (- S). The reaction has a positive G and is, therefore, spontaneous.
D)The reaction is endothermic (- H)as demonstrated by the warm reaction vessel and entropy is increased (- S). The reaction has a positive G and is, therefore, spontaneous.
A)The reaction is exothermic (- H)as demonstrated by the warm reaction vessel and entropy is increased (+ S). The reaction has a negative G and is, therefore, spontaneous.
B)The reaction is exothermic (+ H)as demonstrated by the warm reaction vessel and entropy is increased (+ S). The reaction therefore has a negative G and is, therefore, spontaneous.
C)The reaction is exothermic (+ H)as demonstrated by the warm reaction vessel and entropy is increased (- S). The reaction has a positive G and is, therefore, spontaneous.
D)The reaction is endothermic (- H)as demonstrated by the warm reaction vessel and entropy is increased (- S). The reaction has a positive G and is, therefore, spontaneous.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
9
When energy is being stored for later use or for use at a distant site, the most frequent form of storage is _____________________.
A)as mechanical energy
B)within the chemical bonds of molecules
C)as kinetic energy
D)as electrostatic energy
A)as mechanical energy
B)within the chemical bonds of molecules
C)as kinetic energy
D)as electrostatic energy

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
10
Which choice below correctly rationalized whether this pair of reactions is coupled?
A + B --> C + D ( G = +5.4 kcal/mole)
D + F --> G + H ( G = -4.4 kcal/mole)
A)The equations above do not represent coupled reactions because the overall G is positive.
B)The equations above do not represent coupled reactions because the overall G is negative.
C)The equations represent coupled reactions because the overall G is positive.
D)The equations represent coupled reactions because the overall G is negative.
A + B --> C + D ( G = +5.4 kcal/mole)
D + F --> G + H ( G = -4.4 kcal/mole)
A)The equations above do not represent coupled reactions because the overall G is positive.
B)The equations above do not represent coupled reactions because the overall G is negative.
C)The equations represent coupled reactions because the overall G is positive.
D)The equations represent coupled reactions because the overall G is negative.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
11
The first law of thermodynamics states that ________________:
A)energy can be created and transduced from one form to another
B)energy can be destroyed and can be transduced from one form to another
C)energy cannot be created or destroyed and cannot be transduced from one form to another
D)energy cannot be created or destroyed but can be transduced from one form to another
A)energy can be created and transduced from one form to another
B)energy can be destroyed and can be transduced from one form to another
C)energy cannot be created or destroyed and cannot be transduced from one form to another
D)energy cannot be created or destroyed but can be transduced from one form to another

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
12
What is an essential difference between thermodynamics and bioenergetics?
A)Bioenergetics studies energy transformations taking place within living organisms while thermodynamics studies energy changes within a broader system, up to the size of the universe.
B)Bioenergetics studies energy transformations taking place within living organisms while thermodynamics studies molecular reactions occurring in isolation.
C)Thermodynamics studies energy transformations taking place within living organisms while bioenergetics studies energy changes within a broader system, up to the size of the universe.
D)Bioenergetics studies energy transformations taking place within living organisms while thermodynamics studies only the energy changes of the non-living world.
A)Bioenergetics studies energy transformations taking place within living organisms while thermodynamics studies energy changes within a broader system, up to the size of the universe.
B)Bioenergetics studies energy transformations taking place within living organisms while thermodynamics studies molecular reactions occurring in isolation.
C)Thermodynamics studies energy transformations taking place within living organisms while bioenergetics studies energy changes within a broader system, up to the size of the universe.
D)Bioenergetics studies energy transformations taking place within living organisms while thermodynamics studies only the energy changes of the non-living world.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
13
Which scenario does NOT describe an increase in entropy within the system described?
A)dissolving table salt in warm water
B)running a steam engine on a cold day
C)observing an ice cube on a hot summer's day
D)observing an ice cube at absolute 0 K
A)dissolving table salt in warm water
B)running a steam engine on a cold day
C)observing an ice cube on a hot summer's day
D)observing an ice cube at absolute 0 K

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
14
A pharmacist is looking for a drug which will prevent an enzyme from working by binding to it as an inhibitor. Which of the inhibitors listed as choices will prove most effective, given equality in other considerations such as solubility, toxicity and stability?
A)Substance X, Kd = 2.7
B)Substance Y, Kd = 23
C)Substance Z, Kd = 0.05
D)Substance P, Kd = 0.001
A)Substance X, Kd = 2.7
B)Substance Y, Kd = 23
C)Substance Z, Kd = 0.05
D)Substance P, Kd = 0.001

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
15
You mix reagents (A, B, C, D)so that each is present at an initial concentration of 0.5M. The equilibrium constant for the reaction Keq is 4. Which choice represents the final concentrations of each reagent once equilibrium is achieved?
A)[ 0.67][0.67] [ 0.33][0.33]
B)[ 0.5][0.5] [ 0.25][0.25]
C)[ 0.4][0.4] [ 0.5][0.5]
D)[ 0.4][0.4] [ 0.1][0.1]
A)[ 0.67][0.67] [ 0.33][0.33]
B)[ 0.5][0.5] [ 0.25][0.25]
C)[ 0.4][0.4] [ 0.5][0.5]
D)[ 0.4][0.4] [ 0.1][0.1]

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
16
Entropy is associated with the _________ movement of particles of matter, which because they are ________ cannot accomplish a directed work process.
A)rapid, directed
B)random, random
C)rapid, random
D)slow, rapid
E)random, slow
A)rapid, directed
B)random, random
C)rapid, random
D)slow, rapid
E)random, slow

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the equation sets represents coupled reactions?
A)A + B --> C + D ( G = -5.4 kcal/mole)and E + F --> G + H ( G = +4.4 kcal/mole)
B)A + B --> C + D ( G = -5.4 kcal/mole)and C + D --> E + F ( G = +4.4 kcal/mole)
C)A + B --> C + D ( G = -5.4 kcal/mole)and E + F --> C + D ( G = +4.4 kcal/mole)
D)all are correct examples of coupled reactions
A)A + B --> C + D ( G = -5.4 kcal/mole)and E + F --> G + H ( G = +4.4 kcal/mole)
B)A + B --> C + D ( G = -5.4 kcal/mole)and C + D --> E + F ( G = +4.4 kcal/mole)
C)A + B --> C + D ( G = -5.4 kcal/mole)and E + F --> C + D ( G = +4.4 kcal/mole)
D)all are correct examples of coupled reactions

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
18
A reaction vessel is cold to the touch and the reaction results in an increase in order of the products inside the reaction vessel. Which statement correctly confirms whether or not the reaction is spontaneous and explains why?
A)The reaction is exothermic (- H)as demonstrated by the cold reaction vessel and entropy is increased (+ S). The reaction has a negative G and is, therefore, spontaneous.
B)The reaction is endothermic (+ H)as demonstrated by the cold reaction vessel and entropy is decreased (- S). The reaction therefore has a positive G and is, therefore nonspontaneous.
C)The reaction is exothermic (+ H)as demonstrated by the cold reaction vessel and entropy is increased (- S). The reaction has a positive G and is, therefore, spontaneous.
D)The reaction is endothermic (- H)as demonstrated by the cold reaction vessel and entropy is increased (+ S). The reaction has a positive G and is, therefore, nonspontaneous.
A)The reaction is exothermic (- H)as demonstrated by the cold reaction vessel and entropy is increased (+ S). The reaction has a negative G and is, therefore, spontaneous.
B)The reaction is endothermic (+ H)as demonstrated by the cold reaction vessel and entropy is decreased (- S). The reaction therefore has a positive G and is, therefore nonspontaneous.
C)The reaction is exothermic (+ H)as demonstrated by the cold reaction vessel and entropy is increased (- S). The reaction has a positive G and is, therefore, spontaneous.
D)The reaction is endothermic (- H)as demonstrated by the cold reaction vessel and entropy is increased (+ S). The reaction has a positive G and is, therefore, nonspontaneous.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
19
What kind of organism reaches equilibrium?
A)one that is actively metabolizing
B)one with a low metabolic rate
C)a dead organism
D)a eukaryote
E)a prokaryote
A)one that is actively metabolizing
B)one with a low metabolic rate
C)a dead organism
D)a eukaryote
E)a prokaryote

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
20
The chemical energy stored in ATP is converted to mechanical energy that can move organelles around within the cell. This is an example of __________.
A)being exothermic
B)being endothermic
C)energy transduction
D)polymerization
E)catheterization
A)being exothermic
B)being endothermic
C)energy transduction
D)polymerization
E)catheterization

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
21
In the absence of an enzyme, a monosaccharide like galactose or glucose, will likely decompose in roughly ___________.
A)30 seconds
B)3 weeks
C)30 days
D)30,000 years
A)30 seconds
B)3 weeks
C)30 days
D)30,000 years

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
22
Who was the first scientist to determine the structure and composition of an enzyme?
A)von Liebig
B)Pasteur
C)Sumner
D)Büchner
A)von Liebig
B)Pasteur
C)Sumner
D)Büchner

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
23
What kind of interaction is NOT involved in the binding of a substrate to a normally functioning enzyme?
A)hydrogen bonds
B)a transient covalent bond
C)ionic bonds
D)a permanent covalent bond
E)hydrophobic interactions
A)hydrogen bonds
B)a transient covalent bond
C)ionic bonds
D)a permanent covalent bond
E)hydrophobic interactions

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these antibiotics does NOT target protein synthesis?
A)macrolides
B)lincosamides
C)tetracyclines
D)quinolones
A)macrolides
B)lincosamides
C)tetracyclines
D)quinolones

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
25
What is the effect of a competitive inhibitor on an enzyme-mediated reaction?
A)Vmax stays the same, KM decreases
B)Vmax decreases, KM is decreased
C)Vmax increases, KM is unchanged
D)Vmax stays the same, KM is unchanged
E)Vmax stays the same, KM increases
A)Vmax stays the same, KM decreases
B)Vmax decreases, KM is decreased
C)Vmax increases, KM is unchanged
D)Vmax stays the same, KM is unchanged
E)Vmax stays the same, KM increases

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
26
Enzymes work by ___________.
A)raising the activation energy of a reaction and thus speeding up the reaction
B)lowering the activation energy of a reaction and thus speeding up the reaction
C)raising the G of a reaction and thus speeding up the reaction
D)lowering the G of a reaction and thus speeding up the reaction
E)changing the free energy of the products and thus speeding up the reaction
A)raising the activation energy of a reaction and thus speeding up the reaction
B)lowering the activation energy of a reaction and thus speeding up the reaction
C)raising the G of a reaction and thus speeding up the reaction
D)lowering the G of a reaction and thus speeding up the reaction
E)changing the free energy of the products and thus speeding up the reaction

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
27
The effect of a competitive inhibitor can be reversed by _______.
A)increasing inhibitor concentration
B)increasing substrate concentration
C)heating the reaction mixture
D)changing the pH
E)massaging the enzyme
A)increasing inhibitor concentration
B)increasing substrate concentration
C)heating the reaction mixture
D)changing the pH
E)massaging the enzyme

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
28
In the reaction A + B <-> C + D, how might the reaction take place in the cell if the G is very positive?
A)The reaction would probably take place by coupling it to a reaction with a larger - G so that when the G values are added up the sum is negative.
B)The reaction would probably take place by coupling it to a reaction with a smaller - G so that when the G values are added up the sum is positive.
C)The reaction would probably take place by coupling it to a reaction with a larger G so that when the G values are added up the sum is positive.
D)The reaction would probably take place by coupling it to a reaction with the same positive G so that when the G values are added up the energy levels balance.
A)The reaction would probably take place by coupling it to a reaction with a larger - G so that when the G values are added up the sum is negative.
B)The reaction would probably take place by coupling it to a reaction with a smaller - G so that when the G values are added up the sum is positive.
C)The reaction would probably take place by coupling it to a reaction with a larger G so that when the G values are added up the sum is positive.
D)The reaction would probably take place by coupling it to a reaction with the same positive G so that when the G values are added up the energy levels balance.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
29
Which property below is NOT a characteristic of enzymes?
A)They are only effective in high concentrations.
B)They are altered reversibly during a reaction.
C)They do not alter the ΔG of a reaction.
D)They are used over and over again.
E)They do not determine whether a reaction is exergonic or endergonic.
A)They are only effective in high concentrations.
B)They are altered reversibly during a reaction.
C)They do not alter the ΔG of a reaction.
D)They are used over and over again.
E)They do not determine whether a reaction is exergonic or endergonic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
30
Strategies to combat antimicrobial drug resistance include all of the following EXCEPT:
A)administering more than one antimicrobial drug concurrently
B)designing drugs that avoid interacting with highly conserved areas of their target molecules
C)isolating patients who are diagnosed with antibiotic resistant pathogens
D)observing stricter hygienic practices when caring for those who are diagnosed with antibiotic resistant pathogens
A)administering more than one antimicrobial drug concurrently
B)designing drugs that avoid interacting with highly conserved areas of their target molecules
C)isolating patients who are diagnosed with antibiotic resistant pathogens
D)observing stricter hygienic practices when caring for those who are diagnosed with antibiotic resistant pathogens

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
31
Energy rich monosaccharides and other biological monomers which are seen to exist stably in purified form are said to be:
A)neither thermodynamically nor kinetically stable
B)both thermodynamically and kinetically stable
C)thermodynamically stable but kinetically unstable
D)thermodynamically unstable but kinetically stable
A)neither thermodynamically nor kinetically stable
B)both thermodynamically and kinetically stable
C)thermodynamically stable but kinetically unstable
D)thermodynamically unstable but kinetically stable

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
32
Penicillin fits into the active site of transpeptidases and acts as what kind of inhibitor?
A)irreversible and noncompetitive
B)reversible only
C)competitive and irreversible
D)reversible and noncompetitive
E)None of these are correct.
A)irreversible and noncompetitive
B)reversible only
C)competitive and irreversible
D)reversible and noncompetitive
E)None of these are correct.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
33
You are observing an enzyme driven reaction. You add chemical X, which inhibits the reaction. If you add more substrate, the reaction rate approaches the Vmax of the uninhibited reaction. Furthermore, the structure of X is similar to the natural substrate. What kind of inhibitor is X?
A)irreversible
B)reversible
C)competitive
D)reversible and uncompetitive
E)None of these are correct.
A)irreversible
B)reversible
C)competitive
D)reversible and uncompetitive
E)None of these are correct.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
34
What is the effect of a competitive inhibitor on an enzyme-mediated reaction?
A)Vmax stays the same, KM decreases
B)Vmax decreases, KM is unchanged
C)Vmax increases, KM is unchanged
D)Vmax stays the same, KM is unchanged
E)Vmax stays the same, KM increases
A)Vmax stays the same, KM decreases
B)Vmax decreases, KM is unchanged
C)Vmax increases, KM is unchanged
D)Vmax stays the same, KM is unchanged
E)Vmax stays the same, KM increases

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
35
What molecule type other than protein has been observed to have catalytic activity?
A)carbohydrates
B)lipids
C)RNA
D)DNA
A)carbohydrates
B)lipids
C)RNA
D)DNA

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
36
Doubling the concentration of enzyme will ______ the Vmax and _____ the KM.
A)double, not alter
B)not alter, double
C)double, double
D)not change, not alter
E)halve, halve
A)double, not alter
B)not alter, double
C)double, double
D)not change, not alter
E)halve, halve

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
37
You are a researcher trying to design an enzyme inhibitor for a particular enzyme. Which molecule should your inhibitor most closely resemble for the most effective inhibition to take place?
A)the enzyme's final product
B)the enzyme's initial substrate
C)the transition state intermediate produced during enzyme-substrate interaction
D)the transition state intermediate produced during enzyme-product release
A)the enzyme's final product
B)the enzyme's initial substrate
C)the transition state intermediate produced during enzyme-substrate interaction
D)the transition state intermediate produced during enzyme-product release

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
38
The existence of ________ motion in proteins suggests that enzymes are capable - even in the absence of substrate - of many of the same movements that can be detected during their catalytic cycle.
A)extrinsic
B)intrinsic
C)instant
D)built-in
E)intrinsic and built-in
A)extrinsic
B)intrinsic
C)instant
D)built-in
E)intrinsic and built-in

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
39
What kind of inhibitor binds very tightly to an enzyme often forming a covalent bond with an amino acid in the active site?
A)irreversible
B)reversible
C)uncompetitive
D)reversible and uncompetitive
E)None of these are correct.
A)irreversible
B)reversible
C)uncompetitive
D)reversible and uncompetitive
E)None of these are correct.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
40
The environment within an enzyme's active site tends to have ______________ characteristics.
A)acidic
B)hydrophilic
C)basic
D)hydrophobic
A)acidic
B)hydrophilic
C)basic
D)hydrophobic

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
41
What kind of enzyme adds phosphate groups to enzymes for the purpose of activating or deactivating them?
A)phosphatases
B)protein kinases
C)flippases
D)glycosyltransferases
E)carboxypeptidase
A)phosphatases
B)protein kinases
C)flippases
D)glycosyltransferases
E)carboxypeptidase

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
42
Metabolic pathways that provide building blocks from which other molecules can be synthesized and that provide the chemical energy required for many cell activities are known as ______ reactions.
A)anabolic
B)catabolic
C)allosteric
D)anabolic and catabolic
A)anabolic
B)catabolic
C)allosteric
D)anabolic and catabolic

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
43
Below is a segment of a cell's collection of biochemical pathways. M is a product of one series of these reactions. It is also a regulatory molecule. Look at the pathway below and indicate the position(s)at which M is most likely to act as a feedback inhibitor when its concentration gets too high. 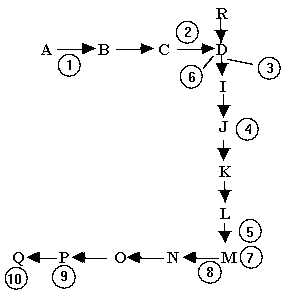
A)Positions 1, 2, 8
B)Positions 4, 5, 7
C)Positions 1, 2, 3
D)Positions 8, 9, 10

A)Positions 1, 2, 8
B)Positions 4, 5, 7
C)Positions 1, 2, 3
D)Positions 8, 9, 10

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
44
Glycolysis occurs in the ________; the Krebs (TCA)cycle occurs in the ______ of eukaryotes and the ______ of prokaryotes.
A)cytoplasm, cytoplasm, cytoplasm
B)mitochondria, cytoplasm, mitochondria
C)cytoplasm, mitochondria, cytoplasm
D)cytoplasm, photosynthesis, cytoplasm
E)cytoplasm, mitochondria, mitochondria
A)cytoplasm, cytoplasm, cytoplasm
B)mitochondria, cytoplasm, mitochondria
C)cytoplasm, mitochondria, cytoplasm
D)cytoplasm, photosynthesis, cytoplasm
E)cytoplasm, mitochondria, mitochondria

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
45
You are studying metabolic pathways and discover that two pathways intersect so that the enzyme basinase participates in both of the intersecting pathways, in one case using substrate K and in the other using substrate M. When presented with substrate K in amounts significantly larger than M, basinase converts K to L which leads eventually to the production of the end product R. The activity of the second pathway is depressed simultaneously. In the presence of large amounts of substrate M and lower amounts of substrate K, the second pathway is activated and culminates in the production of that pathway's end product Y. The activity of the first pathway is depressed simultaneously. What are the alternative substrates K and M acting like?
A)noncompetitive inhibitors
B)competitive inhibitors
C)irreversible inhibitors
D)noncompetitive and irreversible inhibitors
A)noncompetitive inhibitors
B)competitive inhibitors
C)irreversible inhibitors
D)noncompetitive and irreversible inhibitors

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
46
The first exergonic reaction of glycolysis creates:
A)Glucose 6-phosphate
B)3-phosphoglycerate
C)pyruvate
D)dihydroxyacetone phosphate
A)Glucose 6-phosphate
B)3-phosphoglycerate
C)pyruvate
D)dihydroxyacetone phosphate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
47
In glycolysis, fructose 1,6-biphosphate is split into two different molecules with distinct structures. Which enzyme is responsible for converting one of those molecules to be the same as the other?
A)hexokinase
B)phosphofructokinase
C)triose phosphate isomerase
D)enolase
A)hexokinase
B)phosphofructokinase
C)triose phosphate isomerase
D)enolase

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
48
A reaction involving the gain of one or more electrons is _________ reaction.
A)an oxidation
B)a reduction
C)an inclusion
D)an elimination
A)an oxidation
B)a reduction
C)an inclusion
D)an elimination

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
49
Although the glycolytic pathway is considered catabolic in purpose, some of the reactions within the pathway could be considered anabolic. Select the enzyme in glycolysis which catalyses an anabolic reaction.
A)hexokinase
B)phosphoglucose isomerase
C)aldolase
D)phosphoglycerate kinase
A)hexokinase
B)phosphoglucose isomerase
C)aldolase
D)phosphoglycerate kinase

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
50
If an antibiotic were found to bind to a site on an essential bacterial enzyme other than the active site, what would its most likely mode of action be?
A)competitive inhibitor
B)noncompetitive inhibitor
C)inhibitor of protein synthesis
D)lowering activation energy
A)competitive inhibitor
B)noncompetitive inhibitor
C)inhibitor of protein synthesis
D)lowering activation energy

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
51
Given the ability of NADH to transfer energy from the high energy electrons it carries, it is a molecule most analogous to:
A)glucose
B)ADP
C)ATP
D)pyruvate
A)glucose
B)ADP
C)ATP
D)pyruvate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
52
Possible fermentation products include all of the following EXCEPT:
A)lactate produced in mammalian muscle cells
B)ethanol produced in mammalian muscle cells
C)ethanol produced in yeast cells
D)ATP produced in yeast cells
A)lactate produced in mammalian muscle cells
B)ethanol produced in mammalian muscle cells
C)ethanol produced in yeast cells
D)ATP produced in yeast cells

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
53
Although the glycolytic pathway is considered catabolic in purpose, some of the reactions within the pathway could be considered anabolic. Select the enzyme in glycolysis which catalyses an anabolic reaction.
A)phosphofructokinase
B)phosphoglucose isomerase
C)aldolase
D)phosphoglycerate kinase
A)phosphofructokinase
B)phosphoglucose isomerase
C)aldolase
D)phosphoglycerate kinase

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
54
An enzyme has a KM of 20 µM and a Vmax of 50 mmoles of product/minute/µg of enzyme. After exposure to an inhibitor and analysis on a Lineweaver-Burk plot the following values are obtained: -1/ KM = - 0.05 liters/µmole and 1/ Vmax = 0.04 (mmoles of product/minute/µg of enzyme)-1. What kind of inhibitor was used in the experiment and what data interpretation supports this conclusion?
A)noncompetitive inhibitor because the KM has remained the same and the Vmax has decreased
B)competitive inhibitor because the KM has remained the same and the Vmax has decreased
C)noncompetitive inhibitor because the KM has increased and the Vmax has decreased
D)competitive inhibitor because the KM has increased and the Vmax has decreased
A)noncompetitive inhibitor because the KM has remained the same and the Vmax has decreased
B)competitive inhibitor because the KM has remained the same and the Vmax has decreased
C)noncompetitive inhibitor because the KM has increased and the Vmax has decreased
D)competitive inhibitor because the KM has increased and the Vmax has decreased

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
55
An enzyme which engages in an oxidation-reduction activity, transferring high energy electron and protons from a substrate onto a cofactor, is termed ____________________.
A)a kinase
B)a dehydrogenase
C)an isomerase
D)a hydrolase
A)a kinase
B)a dehydrogenase
C)an isomerase
D)a hydrolase

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
56
What is the activity of dehydrogenase enzymes destined to provide in a cell capable of respiration?
A)increased ATP through NADH oxidation
B)decreased ATP through NADH oxidation
C)increased ATP through NADH reduction
D)decreased ATP through NADH reduction
A)increased ATP through NADH oxidation
B)decreased ATP through NADH oxidation
C)increased ATP through NADH reduction
D)decreased ATP through NADH reduction

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
57
An enzyme has a KM of 20 µM and a Vmax of 50 mmoles of product/minute/µg of enzyme. After exposure to an inhibitor and analysis on a Lineweaver-Burk plot the following values are obtained: -1/ KM = - 0.03 liters/µmole and 1/ Vmax = 0.02 (mmoles of product/minute/µg of enzyme)-1. What kind of inhibitor was used in the experiment and what data interpretation supports this conclusion?
A)noncompetitive inhibitor because the KM has remained the same and the Vmax has decreased
B)competitive inhibitor because the KM has remained the same and the Vmax has decreased
C)noncompetitive inhibitor because the KM has increased and the Vmax has decreased
D)competitive inhibitor because the KM has increased and the Vmax remains the same
A)noncompetitive inhibitor because the KM has remained the same and the Vmax has decreased
B)competitive inhibitor because the KM has remained the same and the Vmax has decreased
C)noncompetitive inhibitor because the KM has increased and the Vmax has decreased
D)competitive inhibitor because the KM has increased and the Vmax remains the same

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
58
Which factors distinguish substrate-level phosphorylation from oxidative phosphorylation? Select the INCORRECT response.
A)substrate-level phosphorylation is catalyzed by an enzyme while oxidative phosphorylation requires an electron transport chain
B)substrate-level phosphorylation uses ATP as the source of the phosphate group while oxidative phosphorylation uses Pi
C)substrate-level phosphorylation uses a glycolytic intermediate as the source of the phosphate group while oxidative phosphorylation uses Pi
D)substrate-level phosphorylation takes place in an aqueous environment while oxidative phosphorylation requires a hydrophobic environment.
A)substrate-level phosphorylation is catalyzed by an enzyme while oxidative phosphorylation requires an electron transport chain
B)substrate-level phosphorylation uses ATP as the source of the phosphate group while oxidative phosphorylation uses Pi
C)substrate-level phosphorylation uses a glycolytic intermediate as the source of the phosphate group while oxidative phosphorylation uses Pi
D)substrate-level phosphorylation takes place in an aqueous environment while oxidative phosphorylation requires a hydrophobic environment.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
59
The hydrolysis of one glucose molecule has the potential to produce _______ ATP molecules in cells evolved to optimally catabolize it.
A)5
B)10
C)36
D)120
A)5
B)10
C)36
D)120

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
60
Phosphorylation of glucose by hexokinase and phosphofructokinase is advantageous because:
A)the sugar now has less activation energy
B)a phosphorylated sugar is more likely to diffuse into the environment outside the cell
C)glucose from outside the cell will continue to move into the cytoplasm of the cell
D)all of these statements are correct
A)the sugar now has less activation energy
B)a phosphorylated sugar is more likely to diffuse into the environment outside the cell
C)glucose from outside the cell will continue to move into the cytoplasm of the cell
D)all of these statements are correct

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
61
In cells relying exclusively upon glycolysis and substrate-level phosphorylation for ATP production, why is the production of reduced intermediates from pyruvate so essential?
A)Reduction of pyruvate also allows for the reduction of NADH, providing NAD+ which is an essential substrate for the activity of glyceraldehyde phosphate dehydrogenase.
B)Reduction of pyruvate allows for the oxidation of NADH, providing NAD+ which is an essential substrate for the activity of glyceraldehyde phosphate dehydrogenase.
C)Reduction of pyruvate also allows for the reduction of NADH, providing NAD+ which is an essential substrate for the activity of triose phosphate isomerase.
D)Reduction of pyruvate allows for the oxidation of NADH, providing NAD+ which is an essential substrate for the activity of triose phosphate isomerase.
A)Reduction of pyruvate also allows for the reduction of NADH, providing NAD+ which is an essential substrate for the activity of glyceraldehyde phosphate dehydrogenase.
B)Reduction of pyruvate allows for the oxidation of NADH, providing NAD+ which is an essential substrate for the activity of glyceraldehyde phosphate dehydrogenase.
C)Reduction of pyruvate also allows for the reduction of NADH, providing NAD+ which is an essential substrate for the activity of triose phosphate isomerase.
D)Reduction of pyruvate allows for the oxidation of NADH, providing NAD+ which is an essential substrate for the activity of triose phosphate isomerase.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
62
Fluorescein 18 emission from tumor cells is detected by _________________ detector.
A)an X-ray
B)a photon
C)a positron
D)an electron
A)an X-ray
B)a photon
C)a positron
D)an electron

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
63
Why has the pharmaceutical industry drastically cut resources devoted to the development of new antibiotics? (Select all correct choices)
A)The pharmaceutical industry views the period of time antibiotics need to be taken as too long, making it difficult to keep supply and demand in balance.
B)The pharmaceutical industry views the period of time antibiotics need to be taken as too short, making it difficult to maintain profitability.
C)Pharmaceutical companies view the inevitability of microbial resistance acquisition as a barrier to profitability since drugs that take a long time to produce will have a short market life.
D)Pharmaceutical companies view the holding back of drugs of last resort, such as vancomycin, as sufficient to shore up the problem of drug resistance acquisition.
A)The pharmaceutical industry views the period of time antibiotics need to be taken as too long, making it difficult to keep supply and demand in balance.
B)The pharmaceutical industry views the period of time antibiotics need to be taken as too short, making it difficult to maintain profitability.
C)Pharmaceutical companies view the inevitability of microbial resistance acquisition as a barrier to profitability since drugs that take a long time to produce will have a short market life.
D)Pharmaceutical companies view the holding back of drugs of last resort, such as vancomycin, as sufficient to shore up the problem of drug resistance acquisition.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
64
Tumor imaging can exploit soft tissue differences between normal and malignant cells by using all of the following EXCEPT:
A)X-rays
B)CAT scans
C)infrared light
D)positron emission
A)X-rays
B)CAT scans
C)infrared light
D)positron emission

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
65
2-[18F] fluoro-2-deoxy-D-glucose (FDG)accumulates within tumor cells engaging in higher than normal glycolytic activity. Which reactive functional group is missing in FDG, permitting its accumulation in tumor cells and their detection through PET scans?
A)a methyl group
B)a fluoride atom
C)a phosphate group
D)a hydroxyl group
A)a methyl group
B)a fluoride atom
C)a phosphate group
D)a hydroxyl group

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
66
Advantages derived from the use of a circadian oscillator regulatory mechanism include:
A)less down regulation of proteins needed for photosynthesis during hours of total darkness
B)reduced synthesis of essential photosynthesis proteins on cloudy days
C)reduced synthesis of essential photosynthesis proteins at dawn and dusk
D)prediction of need for photosynthesis-related molecules to maximize photosynthesis during daylight hours
A)less down regulation of proteins needed for photosynthesis during hours of total darkness
B)reduced synthesis of essential photosynthesis proteins on cloudy days
C)reduced synthesis of essential photosynthesis proteins at dawn and dusk
D)prediction of need for photosynthesis-related molecules to maximize photosynthesis during daylight hours

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
67
Evidence suggests that lower levels of insulin in the blood may be important in promoting longevity. Which animal models have been used to gather this evidence? (Select all that apply)
A)mice
B)nematodes
C)humans
D)fruit flies
A)mice
B)nematodes
C)humans
D)fruit flies

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
68
Other than controlling the synthesis of photosynthesis-related proteins and other factors, which process has been observed in plants to be regulated by the circadian oscillator?
A)flower production
B)pollen release
C)leaf curling
D)root production
A)flower production
B)pollen release
C)leaf curling
D)root production

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
69
Which statements regarding the diagnosis and detection of brain tumors are INCORRECT? (Select all incorrect choices)
A)PET scanners will directly detect positrons emitted from fluorescein 18 incorporated into 2-deoxy- D-glucose
B)PET scanners will indirectly detect positrons emitted from fluorescein 18 incorporated into 2-deoxy- D-glucose
C)Tumor localization within the skull relies on tracking a pair of positrons back to the point where they diverged in position
D)Tumor localization within the skull relies on tracking a pair of photons back to the point where they diverged in position
A)PET scanners will directly detect positrons emitted from fluorescein 18 incorporated into 2-deoxy- D-glucose
B)PET scanners will indirectly detect positrons emitted from fluorescein 18 incorporated into 2-deoxy- D-glucose
C)Tumor localization within the skull relies on tracking a pair of positrons back to the point where they diverged in position
D)Tumor localization within the skull relies on tracking a pair of photons back to the point where they diverged in position

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
70
Using an imaging technique such as positron emission tomography (PET)to detect cancer has all the advantages described EXCEPT:
A)the image will be clearer than one derived from X-rays or CAT scans
B)PET data can quantify the level of metabolic activity possessed by the tumor cells which may be useful in determining the rate of tumor growth
C)PET data can predict the next area likely to be invaded by the tumor as it expands or the region to which stray tumor cells may move (metastasize).
D)PET data can determine the size and shape of the tumor
A)the image will be clearer than one derived from X-rays or CAT scans
B)PET data can quantify the level of metabolic activity possessed by the tumor cells which may be useful in determining the rate of tumor growth
C)PET data can predict the next area likely to be invaded by the tumor as it expands or the region to which stray tumor cells may move (metastasize).
D)PET data can determine the size and shape of the tumor

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
71
If people are kept on diets containing about 25% fewer calories than would be required to maintain their initial body weight, what happens? (Select all correct responses)
A)body temperature is raised
B)insulin levels are lowered
C)LDL cholesterol levels are lower
D)DNA damage increases
A)body temperature is raised
B)insulin levels are lowered
C)LDL cholesterol levels are lower
D)DNA damage increases

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
72
You isolate the enzyme that synthesizes folic acid in bacteria and conduct some enzyme kinetics experiments. You find, not surprisingly, that sulfa drugs inhibit the enzyme's activity. What happens to the Vmax and KM of this enzyme when it is treated with sulfa drugs? (Select all the correct statements relating to this experiment)
A)sulfa drugs are competitive inhibitors of the folic acid synthesis pathway
B)Vmax will stay the same and KM of this enzyme will decrease
C)Vmax will decrease and KM of this enzyme will stay the same
D)Vmax will increase and KM of this enzyme will increase
E)Vmax will stay the same and KM of this enzyme will increase
A)sulfa drugs are competitive inhibitors of the folic acid synthesis pathway
B)Vmax will stay the same and KM of this enzyme will decrease
C)Vmax will decrease and KM of this enzyme will stay the same
D)Vmax will increase and KM of this enzyme will increase
E)Vmax will stay the same and KM of this enzyme will increase

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
73
Fluorescein 18 emits ________________________ as it decays to another isotope.
A)X-rays
B)photons
C)positrons
D)electrons
A)X-rays
B)photons
C)positrons
D)electrons

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
74
If mice are maintained on very strict diets with reduced caloric intake, what happens to their life span as compared to littermates fed diets with normal caloric content and why? (Select all correct responses)
A)mice live 30-40% longer
B)mice experience a raise in body temperature
C)mice synthesize higher concentrations of superoxide dismutase
D)mice experience less free radical damage
A)mice live 30-40% longer
B)mice experience a raise in body temperature
C)mice synthesize higher concentrations of superoxide dismutase
D)mice experience less free radical damage

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
75
Which statements accurately describe the metabolism and detection of tumor cells? (Select all that are correct choices)
A)tumor cells rely more on anaerobic activity than healthy cells
B)tumor cells exhibit the Warburg effect
C)injection of FDG into a cancer patient will allow fluorescent detection of the tumor region
D)PET localization of tumor masses requires a single photon detector
A)tumor cells rely more on anaerobic activity than healthy cells
B)tumor cells exhibit the Warburg effect
C)injection of FDG into a cancer patient will allow fluorescent detection of the tumor region
D)PET localization of tumor masses requires a single photon detector

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
76
When using 2-[18F] fluoro-2-deoxy-D-glucose (FDG)uptake by tumor cells to detect cancerous masses, fluorescein 18 decays releasing __________. This in turn reacts with __________ and releases pairs of _________________ which are ultimately detected by the diagnostic scanner.
A)an electron, a positron, photons
B)a positron, an electron, photons
C)a photon, an electron, positrons
D)a photon, a positron, electrons
A)an electron, a positron, photons
B)a positron, an electron, photons
C)a photon, an electron, positrons
D)a photon, a positron, electrons

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
77
2-[18F] fluoro-2-deoxy-D-glucose (FDG)acts as a competitive inhibitor of ______________.
A)all enzymes in glycolysis
B)phosphoglucose isomerase
C)hexokinase
D)phosphofructokinase
A)all enzymes in glycolysis
B)phosphoglucose isomerase
C)hexokinase
D)phosphofructokinase

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
78
Plants can regulate proteins, chlorophylls and other factors required for photosynthesis via a light/dark independent regulatory mechanism known as a _______________________.
A)regulating oscillator
B)circadian oscillator
C)diurnal oscillator
D)entraining oscillator
A)regulating oscillator
B)circadian oscillator
C)diurnal oscillator
D)entraining oscillator

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
What is responsible for the periodicity that controls the production of essential photosynthetic enzymes and factors through the circadian oscillator?
A)carbohydrate availability
B)lipid levels within the cell
C)a protein network synchronized through light sensitivity
D)an RNA network synchronized through light sensitivity
A)carbohydrate availability
B)lipid levels within the cell
C)a protein network synchronized through light sensitivity
D)an RNA network synchronized through light sensitivity

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
80
If you were comparing circadian oscillator structures between plant species, where might you predict that there would be the greatest differences?
A)between tropical orchids and arctic tundra wildflowers
B)between east and west coast cereal crops grown at the same latitude
C)between spring onion plants from the northern and southern hemisphere grown at the same distances away from the equator (each measured at their spring growth optimum)
D)between plants in a garden grown in either a sunny or a shady spot
A)between tropical orchids and arctic tundra wildflowers
B)between east and west coast cereal crops grown at the same latitude
C)between spring onion plants from the northern and southern hemisphere grown at the same distances away from the equator (each measured at their spring growth optimum)
D)between plants in a garden grown in either a sunny or a shady spot

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck


