Exam 3: Bioenergetics, Enzymes and Metabolism
Exam 1: Introduction to the Study of Cell and Molecular Biology100 Questions
Exam 2: The Chemical Basis of Life87 Questions
Exam 3: Bioenergetics, Enzymes and Metabolism80 Questions
Exam 4: The Structure and Function of the Plasma Membrane99 Questions
Exam 5: Aerobic Respiration and the Mitochondrion99 Questions
Exam 6: Photosynthesis and the Chloroplast100 Questions
Exam 7: Interactions Between Cells and Their Environment103 Questions
Exam 8: Cytoplasmic Membrane Systems: Structure, Function, and Membrane Trafficking159 Questions
Exam 9: The Cytoskeleton and Cell Motility107 Questions
Exam 10: The Nature of the Gene and the Genome97 Questions
Exam 11: Gene Expression: From Transcription to Translation101 Questions
Exam 12: Control of Gene Expression100 Questions
Exam 13: Dna Replication and Repair98 Questions
Exam 14: Cellular Reproduction103 Questions
Exam 15: Cell Signaling and Signal Transduction: Communication Between Cells109 Questions
Exam 16: Cancer98 Questions
Exam 17: The Immune Response109 Questions
Exam 18: Techniques in Cell and Molecular Biology112 Questions
Select questions type
Which choice below correctly rationalized whether this pair of reactions is coupled?
A + B --> C + D ( G = +5.4 kcal/mole)
D + F --> G + H ( G = -4.4 kcal/mole)
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
A
The hydrolysis of one glucose molecule has the potential to produce _______ ATP molecules in cells evolved to optimally catabolize it.
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
If an antibiotic were found to bind to a site on an essential bacterial enzyme other than the active site, what would its most likely mode of action be?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
B
An enzyme which engages in an oxidation-reduction activity, transferring high energy electron and protons from a substrate onto a cofactor, is termed ____________________.
(Multiple Choice)
4.8/5  (38)
(38)
Fluorescein 18 emits ________________________ as it decays to another isotope.
(Multiple Choice)
4.9/5  (34)
(34)
Strategies to combat antimicrobial drug resistance include all of the following EXCEPT:
(Multiple Choice)
4.7/5  (30)
(30)
Which of these antibiotics does NOT target protein synthesis?
(Multiple Choice)
4.9/5  (34)
(34)
An enzyme has a KM of 20 µM and a Vmax of 50 mmoles of product/minute/µg of enzyme. After exposure to an inhibitor and analysis on a Lineweaver-Burk plot the following values are obtained: -1/ KM = - 0.05 liters/µmole and 1/ Vmax = 0.04 (mmoles of product/minute/µg of enzyme)-1. What kind of inhibitor was used in the experiment and what data interpretation supports this conclusion?
(Multiple Choice)
4.7/5  (41)
(41)
Below is a segment of a cell's collection of biochemical pathways. M is a product of one series of these reactions. It is also a regulatory molecule. Look at the pathway below and indicate the position(s)at which M is most likely to act as a feedback inhibitor when its concentration gets too high. 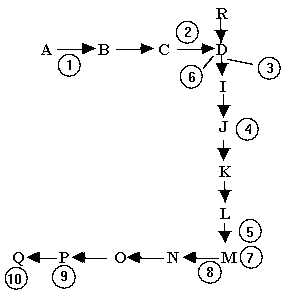
(Multiple Choice)
4.8/5  (28)
(28)
Plants can regulate proteins, chlorophylls and other factors required for photosynthesis via a light/dark independent regulatory mechanism known as a _______________________.
(Multiple Choice)
4.9/5  (32)
(32)
What is responsible for the periodicity that controls the production of essential photosynthetic enzymes and factors through the circadian oscillator?
(Multiple Choice)
4.8/5  (37)
(37)
Although the glycolytic pathway is considered catabolic in purpose, some of the reactions within the pathway could be considered anabolic. Select the enzyme in glycolysis which catalyses an anabolic reaction.
(Multiple Choice)
4.7/5  (33)
(33)
You mix reagents (A, B, C, D)so that each is present at an initial concentration of 0.5M. The equilibrium constant for the reaction Keq is 4. Which choice represents the final concentrations of each reagent once equilibrium is achieved?
(Multiple Choice)
4.9/5  (43)
(43)
Metabolic pathways that provide building blocks from which other molecules can be synthesized and that provide the chemical energy required for many cell activities are known as ______ reactions.
(Multiple Choice)
4.8/5  (27)
(27)
Given the ability of NADH to transfer energy from the high energy electrons it carries, it is a molecule most analogous to:
(Multiple Choice)
4.7/5  (42)
(42)
The effect of a competitive inhibitor can be reversed by _______.
(Multiple Choice)
4.8/5  (38)
(38)
After allowing the reaction to proceed, you find that the the final concentrations of each reagent once equilibrium is achieved is
What is the value of the equilibrium constant Keq?
(Multiple Choice)
4.9/5  (33)
(33)
When energy is being stored for later use or for use at a distant site, the most frequent form of storage is _____________________.
(Multiple Choice)
4.8/5  (21)
(21)
Which statements regarding the diagnosis and detection of brain tumors are INCORRECT? (Select all incorrect choices)
(Multiple Choice)
4.9/5  (29)
(29)
Showing 1 - 20 of 80
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)