Deck 10: The Liquid and Solid States
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 10: The Liquid and Solid States
1
Three phases of water are shown in the figure. List the terms used to identify the phase changes indicated by the arrows. 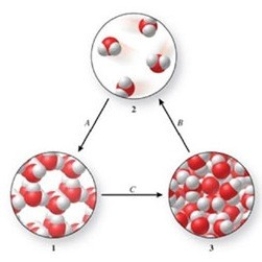
A)A = sublimation, B = vaporization, C = freezing
B)A = condensation, B = sublimation, C = melting
C)A = freezing, B = vaporization, C = melting
D)A = deposition, B = vaporization, C = melting
E)A = deposition, B = sublimation, C = freezing

A)A = sublimation, B = vaporization, C = freezing
B)A = condensation, B = sublimation, C = melting
C)A = freezing, B = vaporization, C = melting
D)A = deposition, B = vaporization, C = melting
E)A = deposition, B = sublimation, C = freezing
A = deposition, B = vaporization, C = melting
2
The pictured change of state occurs at constant temperature even though heat is being added. Where does this change of state occur on the cooling curve? 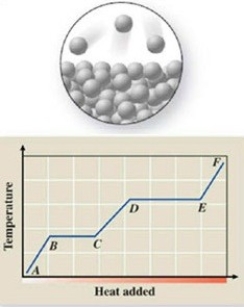
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF
DE
3
What phase change is occurring in the figure, and is it endothermic or exothermic? 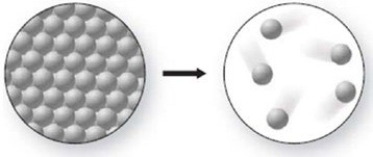
A)melting; exothermic
B)freezing; endothermic
C)freezing; exothermic
D)melting; endothermic
E)sublimation; endothermic

A)melting; exothermic
B)freezing; endothermic
C)freezing; exothermic
D)melting; endothermic
E)sublimation; endothermic
sublimation; endothermic
4
The pictured change of state occurs at constant temperature even though heat is being added. Where does this change of state occur on the cooling curve? 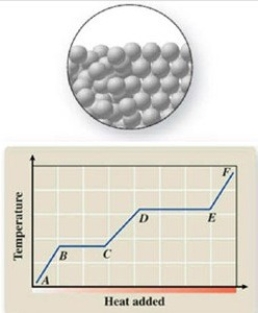
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
The pictured change of state occurs at constant temperature even though heat is being removed. Where does this change of state occur on the cooling curve? 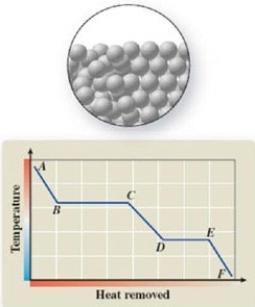
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is correct?
A)At the melting point of a substance, the solid, liquid, and gas phases are all in equilibrium.
B)The freezing point and the melting point of a substance are the same.
C)There is no fundamental difference in the melting process for covalent substances and ionic substances.
D)In order for a substance to go from the gas phase to the liquid phase, it must absorb energy from the surroundings.
E)In order for a substance to go from the liquid phase to the gas phase, it must give off energy to the surroundings.
A)At the melting point of a substance, the solid, liquid, and gas phases are all in equilibrium.
B)The freezing point and the melting point of a substance are the same.
C)There is no fundamental difference in the melting process for covalent substances and ionic substances.
D)In order for a substance to go from the gas phase to the liquid phase, it must absorb energy from the surroundings.
E)In order for a substance to go from the liquid phase to the gas phase, it must give off energy to the surroundings.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
From the vapor pressure curve of propane, it can be seen that the normal boiling point of propane is about ________. 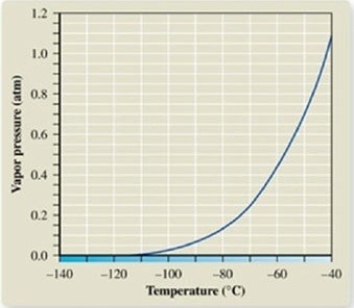
A)−137ºC
B)−120ºC
C)−60ºC
D)−42ºC
E)0ºC

A)−137ºC
B)−120ºC
C)−60ºC
D)−42ºC
E)0ºC

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
The atmospheric pressure on Mount Everest is about 0.30 atm. From the vapor pressure curve of propane, it can be seen the boiling point of propane at this elevation is about ________. 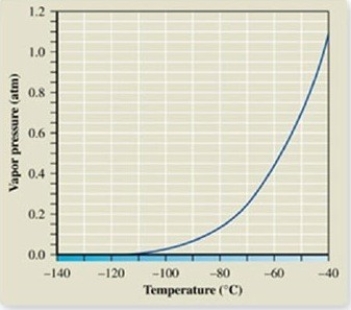
A)−137ºC
B)−120ºC
C)−67ºC
D)−42ºC
E)0ºC

A)−137ºC
B)−120ºC
C)−67ºC
D)−42ºC
E)0ºC

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is correct?
A)When the temperature of a sample of liquid is increased, the molecules move faster, but there is no change in the vapor pressure of the sample.
B)The melting point of metallic sodium is 97.8ºC, so at a temperature of 25ºC, it is a liquid.
C)If you cook an egg on a mountain, it will require a short cooking time, due to the high elevation.
D)When liquids evaporate, it requires an input of energy from the surroundings, so the surroundings become warm.
E)Nitrogen gas has a boiling point of −196ºC, so if it is cooled to −200ºC, it condenses.
A)When the temperature of a sample of liquid is increased, the molecules move faster, but there is no change in the vapor pressure of the sample.
B)The melting point of metallic sodium is 97.8ºC, so at a temperature of 25ºC, it is a liquid.
C)If you cook an egg on a mountain, it will require a short cooking time, due to the high elevation.
D)When liquids evaporate, it requires an input of energy from the surroundings, so the surroundings become warm.
E)Nitrogen gas has a boiling point of −196ºC, so if it is cooled to −200ºC, it condenses.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is correct?
A)The phase change from solid to liquid is exothermic.
B)Liquids boil at lower than normal temperatures in Death Valley, since it is below sea level.
C)A substance with a low boiling point would be expected to have a relatively low vapor pressure at a low temperature.
D)The phase change from liquid to gas is exothermic.
E)When a substance condenses, heat energy is given off.
A)The phase change from solid to liquid is exothermic.
B)Liquids boil at lower than normal temperatures in Death Valley, since it is below sea level.
C)A substance with a low boiling point would be expected to have a relatively low vapor pressure at a low temperature.
D)The phase change from liquid to gas is exothermic.
E)When a substance condenses, heat energy is given off.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements is correct?
A)A liquid which has a high boiling point has a higher vapor pressure at a given temperature than a liquid with a low boiling point.
B)Liquids boil at higher temperatures in the mountains due to the higher altitude.
C)Evaporation is an exothermic process, since heat is given off when a substance vaporizes.
D)Since acetone (nail polish remover)evaporates more readily than water, one would assume that acetone has weaker intermolecular forces.
E)The phase change from solid to gas is exothermic.
A)A liquid which has a high boiling point has a higher vapor pressure at a given temperature than a liquid with a low boiling point.
B)Liquids boil at higher temperatures in the mountains due to the higher altitude.
C)Evaporation is an exothermic process, since heat is given off when a substance vaporizes.
D)Since acetone (nail polish remover)evaporates more readily than water, one would assume that acetone has weaker intermolecular forces.
E)The phase change from solid to gas is exothermic.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
The pictured change of state occurs at constant temperature even though heat is being removed. Where does this change of state occur on the cooling curve? 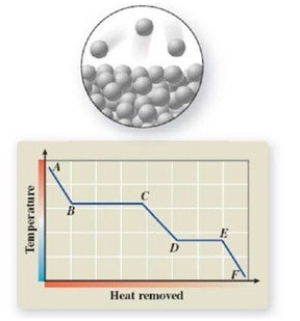
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the molecular-level images in the figure shows the liquid and vapor phases in equilibrium in a closed container? 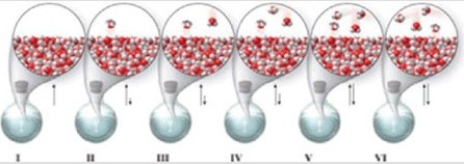
A)I, II, and III
B)none of the images
C)I
D)V
E)V and VI

A)I, II, and III
B)none of the images
C)I
D)V
E)V and VI

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
What phase transition is occurring between points D and E on the cooling curve? 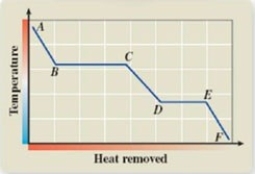
A)melting
B)freezing
C)evaporation
D)sublimation
E)deposition

A)melting
B)freezing
C)evaporation
D)sublimation
E)deposition

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
Three phases of water are shown in the figure. List the terms used to identify the phase changes indicated by the arrows. 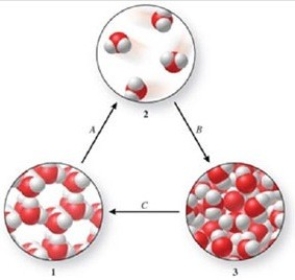
A)A = sublimation, B = vaporization, C = freezing
B)A = vaporization, B = condensation, C = melting
C)A = vaporization, B = deposition, C = melting
D)A = sublimation, B = condensation, C = freezing
E)A = condensation, B = sublimation, C = freezing

A)A = sublimation, B = vaporization, C = freezing
B)A = vaporization, B = condensation, C = melting
C)A = vaporization, B = deposition, C = melting
D)A = sublimation, B = condensation, C = freezing
E)A = condensation, B = sublimation, C = freezing

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
What phase transition is occurring between points B and C on the cooling curve? 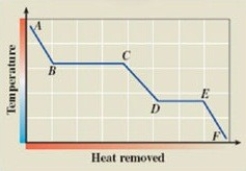
A)melting
B)condensation
C)evaporation
D)sublimation
E)deposition

A)melting
B)condensation
C)evaporation
D)sublimation
E)deposition

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
What phase transition is occurring between points D and E on the heating curve? 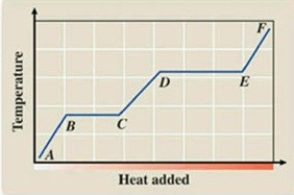
A)melting
B)freezing
C)evaporation
D)sublimation
E)deposition

A)melting
B)freezing
C)evaporation
D)sublimation
E)deposition

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
What phase change is occurring in the figure, and is it endothermic or exothermic? 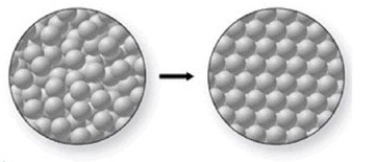
A)melting; exothermic
B)freezing; endothermic
C)freezing; exothermic
D)melting; endothermic
E)condensation; endothermic

A)melting; exothermic
B)freezing; endothermic
C)freezing; exothermic
D)melting; endothermic
E)condensation; endothermic

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
From the vapor pressure curve of acetone, it can be seen that the normal boiling point of acetone is about ________. 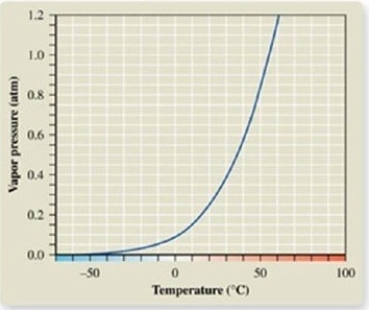
A)-50ºC
B)0ºC
C)50ºC
D)55ºC
E)100ºC

A)-50ºC
B)0ºC
C)50ºC
D)55ºC
E)100ºC

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
What phase transition is occurring between points B and C on the heating curve? 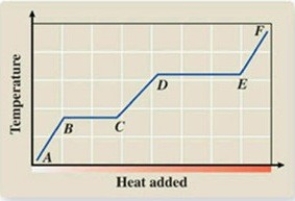
A)melting
B)condensation
C)evaporation
D)sublimation
E)deposition

A)melting
B)condensation
C)evaporation
D)sublimation
E)deposition

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the amount of heat energy, in units of kilojoules, required to evaporate 35.0 g of ethanol, CH3CH2OH, at 78.4ºC, ethanol's boiling point. (The molar heat of vaporization of liquid ethanol is 3.86 × 104 J/mol. The molar mass of ethanol is 46.07 g/mol.)
A)29.3 kJ
B)1.35 × 103 kJ
C)1.10 × 103 kJ
D)8.38 × 102 kJ
E)9.21 × 10−4 kJ
A)29.3 kJ
B)1.35 × 103 kJ
C)1.10 × 103 kJ
D)8.38 × 102 kJ
E)9.21 × 10−4 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements regarding intermolecular forces is incorrect?
A)An intermolecular force is an attractive force that operates between molecules.
B)Bonding forces are much stronger than intermolecular forces.
C)London dispersion forces occur in all atoms and molecules.
D)London dispersion forces are the result of permanent dipoles in atoms or molecules.
E)Molecules which have hydrogen bonded to F, O, or N can undergo hydrogen-bonding.
A)An intermolecular force is an attractive force that operates between molecules.
B)Bonding forces are much stronger than intermolecular forces.
C)London dispersion forces occur in all atoms and molecules.
D)London dispersion forces are the result of permanent dipoles in atoms or molecules.
E)Molecules which have hydrogen bonded to F, O, or N can undergo hydrogen-bonding.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
The physical properties of a substance influenced by the strength of intermolecular forces include all of the following except
A)density.
B)boiling point.
C)surface tension.
D)melting point.
E)mass.
A)density.
B)boiling point.
C)surface tension.
D)melting point.
E)mass.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
The diagram represents the physical state of a substance. Where does this physical state occur on the heating curve? 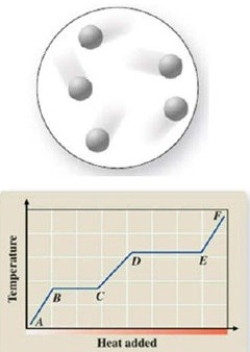
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
Calculate the amount of heat energy required to convert 35.0 g of ice at -12.5°C to water at 24.0°C. (Cwater = 4.184 J/g°C; Cice = 2.03 J/g°C; molar heat of fusion of ice = 6.01 × 103 J/mol)
A)8.88 × 102 J
B)1.17 × 104 J
C)1.61 × 1010 J
D)3.51 × 103 J
E)1.61 × 104 J
A)8.88 × 102 J
B)1.17 × 104 J
C)1.61 × 1010 J
D)3.51 × 103 J
E)1.61 × 104 J

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is not an attractive force that acts between the individual molecules of CH3OH?
A)London dispersion forces
B)dipole-dipole forces
C)hydrogen-bonding forces
D)covalent bonds
E)none of these is correct
A)London dispersion forces
B)dipole-dipole forces
C)hydrogen-bonding forces
D)covalent bonds
E)none of these is correct

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
The diagram represents the physical state of a substance. Where does this physical state occur on the cooling curve? 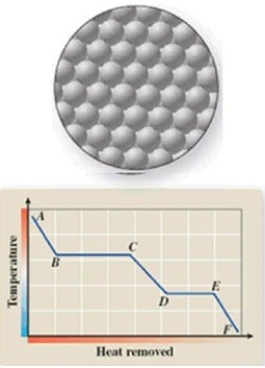
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
The physical properties of a substance influenced by the strength of intermolecular forces include all of the following except
A)melting point.
B)boiling point.
C)viscosity.
D)vapor pressure.
E)mass.
A)melting point.
B)boiling point.
C)viscosity.
D)vapor pressure.
E)mass.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
A)Ni
B)Hg
C)CO2
D)C7H16
E)CBr4
A)Ni
B)Hg
C)CO2
D)C7H16
E)CBr4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the amount of heat energy required to evaporate 27.5 g of water at 100.0ºC. (molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
A)62.1 kJ
B)1.12 × 103 kJ
C)2.02 × 104 kJ
D)6.20 × 107 kJ
E)1.22 × 10−2 kJ
A)62.1 kJ
B)1.12 × 103 kJ
C)2.02 × 104 kJ
D)6.20 × 107 kJ
E)1.22 × 10−2 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the amount of heat energy required to evaporate 55.0 g of water at 100.0ºC. (molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
A)1.24 × 102 kJ
B)2.24 × 103 kJ
C)4.03 × 104 kJ
D)1.24 × 108 kJ
E)2.43 × 10−2 kJ
A)1.24 × 102 kJ
B)2.24 × 103 kJ
C)4.03 × 104 kJ
D)1.24 × 108 kJ
E)2.43 × 10−2 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the amount of heat energy, in units of kilojoules, required to melt 75.0 g of ice at 0.0ºC. (molar heat of fusion of ice = 6.01 × 103 J/mol)
A)4.50 × 102 kJ
B)25.0 kJ
C)13.0 kJ
D)2.25 × 103 kJ
E)4.50 × 10−1 kJ
A)4.50 × 102 kJ
B)25.0 kJ
C)13.0 kJ
D)2.25 × 103 kJ
E)4.50 × 10−1 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the amount of heat energy, in units of kilojoules, required to convert 50.0 g of liquid ethanol, CH3CH2OH, at 25.5°C to gaseous ethanol at 92.0°C. (Cethanol liquid = 2.44 J/g°C; Cethanol gas = 1.42 J/g°C; molar heat of vaporization of liquid ethanol = 3.86 × 104 J/mol. The boiling point of ethanol is 78.4°C and its molar mass is 46.07 g/mol.)
A)49.2 kJ
B)1.93 × 103 kJ
C)0.862 kJ
D)1.35 × 102 kJ
E)2.67 kJ
A)49.2 kJ
B)1.93 × 103 kJ
C)0.862 kJ
D)1.35 × 102 kJ
E)2.67 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
A)Cs
B)SO2
C)I2
D)CCl4
E)Ni
A)Cs
B)SO2
C)I2
D)CCl4
E)Ni

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
The diagram represents the physical state of a substance. Where does this physical state occur on the heating curve? 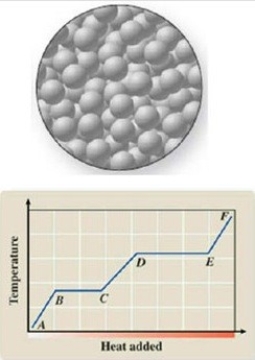
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate the amount of heat energy required to convert 55.0 g of water at 62.5ºC to steam at 124.0°C. (Cwater = 4.184 J/g°C; Csteam = 2.02 J/g°C; molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
A)2.25 × 103 kJ
B)1.23 × 102 kJ
C)0.862 kJ
D)1.36 × 102 kJ
E)2.67 kJ
A)2.25 × 103 kJ
B)1.23 × 102 kJ
C)0.862 kJ
D)1.36 × 102 kJ
E)2.67 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
The diagram represents the physical state of a substance. Where does this physical state occur on the cooling curve? 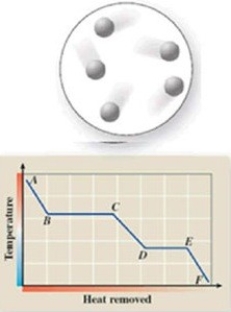
A)AB
B)BC
C)CD
D)DC
E)EF

A)AB
B)BC
C)CD
D)DC
E)EF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the amount of heat energy required to melt 25.0 g of ice at 0.0°C. (molar heat of fusion of ice = 6.01 × 103 J/mol)
A)1.50 × 102 kJ
B)8.34 kJ
C)4.33 kJ
D)7.50 × 102 kJ
E)1.50 × 10−1 kJ
A)1.50 × 102 kJ
B)8.34 kJ
C)4.33 kJ
D)7.50 × 102 kJ
E)1.50 × 10−1 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
A)I2
B)Pb
C)Ar
D)CCl4
E)C8H18
A)I2
B)Pb
C)Ar
D)CCl4
E)C8H18

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the amount of heat energy required to convert 27.5 g of water at 62.5°C to steam at 124.0°C. (Cwater = 4.184 J/g°C; Cice = 2.03 J/gºC; Csteam = 2.02 J/g°C; molar heat of fusion of ice = 6.01 × 103 J/mol; molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
A)6.15 × 104 kJ
B)1.23 × 102 kJ
C)4.31 × 102 kJ
D)67.8 kJ
E)1.33 kJ
A)6.15 × 104 kJ
B)1.23 × 102 kJ
C)4.31 × 102 kJ
D)67.8 kJ
E)1.33 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following molecules experience dipole-dipole forces?
A)NO
B)Cl2
C)CH4
D)SiH4
E)all of these
A)NO
B)Cl2
C)CH4
D)SiH4
E)all of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
Rank the following substances in order of increasing intermolecular forces: Ar, H2O, N2, O2
A)Ar < H2O < N2 < O2
B)Ar < H2O < O2 < N2
C)H2O < N2 < O2 < Ar
D)N2 < O2 < Ar < H2O
E)Ar < N2 < O2 < H2O
A)Ar < H2O < N2 < O2
B)Ar < H2O < O2 < N2
C)H2O < N2 < O2 < Ar
D)N2 < O2 < Ar < H2O
E)Ar < N2 < O2 < H2O

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following has the highest boiling point?
A)H2
B)H2O
C)H2S
D)H2Se
E)H2Te
A)H2
B)H2O
C)H2S
D)H2Se
E)H2Te

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
Rank the following substances in order of increasing boiling point: O2, Ne, Ar, H2
A)O2 < Ne < Ar < H2
B)O2 < Ar < Ne < H2
C)H2 < Ne < Ar < O2
D)H2 < Ne < O2 < Ar
E)Ar < O2 < Ne < H2
A)O2 < Ne < Ar < H2
B)O2 < Ar < Ne < H2
C)H2 < Ne < Ar < O2
D)H2 < Ne < O2 < Ar
E)Ar < O2 < Ne < H2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following has the strongest London dispersion forces?
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
Rank the following substances in order of increasing boiling point: F2, Ne, He, Cl2
A)F2 < Ne < He < Cl2
B)F2 < He < Ne < Cl2
C)F2 < Cl2 < He < Ne
D)He < Ne < F2 < Cl2
E)Cl2 < F2 < Ne < He
A)F2 < Ne < He < Cl2
B)F2 < He < Ne < Cl2
C)F2 < Cl2 < He < Ne
D)He < Ne < F2 < Cl2
E)Cl2 < F2 < Ne < He

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
Rank the following substances in order of increasing boiling point: Cl2, Ar, Ne, Br2
A)Cl2 < Ar < Ne < Br2
B)Ar < Ne < Br2 < Cl2
C)Ne < Ar < Br2 < Cl2
D)Ne < Ar < Cl2 < Br2
E)Cl2 < Br2 < Ar < Ne
A)Cl2 < Ar < Ne < Br2
B)Ar < Ne < Br2 < Cl2
C)Ne < Ar < Br2 < Cl2
D)Ne < Ar < Cl2 < Br2
E)Cl2 < Br2 < Ar < Ne

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the molecules in the figure has hydrogen bonding in the pure liquid state? 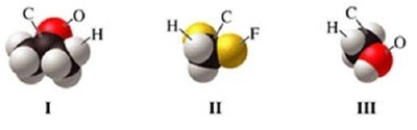
A)I only
B)II only
C)III only
D)II and III
E)I and II

A)I only
B)II only
C)III only
D)II and III
E)I and II

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Rank the following substances in order of increasing intermolecular forces: He, HF, F2, Ne
A)He < HF < F2 < Ne
B)He < HF < Ne < F2
C)He < Ne < F2 < HF
D)He < F2 < Ne < HF
E)HF < Ne < F2 < He
A)He < HF < F2 < Ne
B)He < HF < Ne < F2
C)He < Ne < F2 < HF
D)He < F2 < Ne < HF
E)HF < Ne < F2 < He

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following has the highest boiling point?
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following substances can participate in hydrogen bonding?
A)SiH4
B)CH2Cl2
C)H2O
D)PH3
E)both H2O and PH3
A)SiH4
B)CH2Cl2
C)H2O
D)PH3
E)both H2O and PH3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following substances can participate in hydrogen bonding?
A)CH4
B)CHCl3
C)H2S
D)NH3
E)both H2S and NH3
A)CH4
B)CHCl3
C)H2S
D)NH3
E)both H2S and NH3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following has the highest vapor pressure at a given temperature?
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following molecules experience dipole-dipole forces?
A)NO2
B)CO2
C)CBr4
D)SO3
E)F2
A)NO2
B)CO2
C)CBr4
D)SO3
E)F2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following has the highest boiling point?
A)H2
B)HF
C)HCl
D)HBr
E)HI
A)H2
B)HF
C)HCl
D)HBr
E)HI

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following substances is most likely to be a gas at room temperature?
A)CBr4
B)F2
C)Mn
D)C6H14
E)Sr
A)CBr4
B)F2
C)Mn
D)C6H14
E)Sr

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
Rank the following substances in order of increasing intermolecular forces: Ne, NH3, H2, O2
A)Ne < NH3 < H2 < O2
B)Ne < NH3 < O2 < H2
C)NH3 < O2 < H2 < Ne
D)O2 < H2 < Ne < NH3
E)H2 < Ne < O2 < NH3
A)Ne < NH3 < H2 < O2
B)Ne < NH3 < O2 < H2
C)NH3 < O2 < H2 < Ne
D)O2 < H2 < Ne < NH3
E)H2 < Ne < O2 < NH3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the molecules in the figure has hydrogen bonding in the pure liquid state? 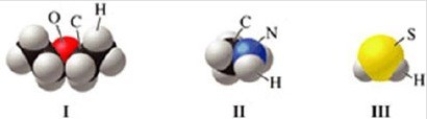
A)I only
B)II only
C)III only
D)II and III
E)I and II

A)I only
B)II only
C)III only
D)II and III
E)I and II

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following substances can participate in hydrogen bonding?
A)CH4
B)HF
C)CH3COCH3
D)SiH4
E)all of these choices are correct
A)CH4
B)HF
C)CH3COCH3
D)SiH4
E)all of these choices are correct

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following molecules experience dipole-dipole forces?
A)CO2
B)SO2
C)CCl4
D)H2S
E)both SO2 and H2S
A)CO2
B)SO2
C)CCl4
D)H2S
E)both SO2 and H2S

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following statements regarding the solid state is incorrect?
A)Most polymers, such as rubbers and plastics, are amorphous.
B)Minerals, such as quartz and amethyst, are crystalline solids.
C)Solids normally do not flow.
D)The particles of a solid are held in a rigid, 3-dimensional array.
E)The arrangement of the particles in a crystal lattice is called space packing.
A)Most polymers, such as rubbers and plastics, are amorphous.
B)Minerals, such as quartz and amethyst, are crystalline solids.
C)Solids normally do not flow.
D)The particles of a solid are held in a rigid, 3-dimensional array.
E)The arrangement of the particles in a crystal lattice is called space packing.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following statements regarding intermolecular forces is incorrect?
A)Hydrogen bonding is the strongest of the intermolecular forces.
B)London dispersion forces increase with increasing molecular size.
C)Dipole-dipole forces are present in all atoms and molecules.
D)In order for a pure substance to participate in hydrogen bonding, it must have hydrogen bonded to F, O, or N.
E)Intermolecular forces are much weaker than bonding forces.
A)Hydrogen bonding is the strongest of the intermolecular forces.
B)London dispersion forces increase with increasing molecular size.
C)Dipole-dipole forces are present in all atoms and molecules.
D)In order for a pure substance to participate in hydrogen bonding, it must have hydrogen bonded to F, O, or N.
E)Intermolecular forces are much weaker than bonding forces.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
Which choice correctly lists the intermolecular forces present in CH3Br?
A)London forces only
B)London forces and dipole-dipole forces
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)London forces and hydrogen bonding
A)London forces only
B)London forces and dipole-dipole forces
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)London forces and hydrogen bonding

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following statements regarding the solid state is incorrect?
A)Many metals follow a close-packing arrangement of their atoms, much like the way fruit is stacked in the grocery store.
B)The forces holding the particles together in a solid will affect their properties.
C)Network solids tend to have very high melting points.
D)Nonpolar molecular solids tend to be good conductors of heat and electricity.
E)Ionic solids tend to be brittle.
A)Many metals follow a close-packing arrangement of their atoms, much like the way fruit is stacked in the grocery store.
B)The forces holding the particles together in a solid will affect their properties.
C)Network solids tend to have very high melting points.
D)Nonpolar molecular solids tend to be good conductors of heat and electricity.
E)Ionic solids tend to be brittle.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements regarding the liquid state is incorrect?
A)Liquids are about 1000 times denser than gases.
B)Liquids are normally about 90-95% as dense as their corresponding solid.
C)The viscosity of a liquid is a measure of its resistance to flow.
D)Molecules with high intermolecular forces will have high surface tension.
E)Surface tension increases as temperature increases.
A)Liquids are about 1000 times denser than gases.
B)Liquids are normally about 90-95% as dense as their corresponding solid.
C)The viscosity of a liquid is a measure of its resistance to flow.
D)Molecules with high intermolecular forces will have high surface tension.
E)Surface tension increases as temperature increases.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is not one of the classifications into which solids can be organized?
A)metallic
B)ionic
C)molecular
D)liquid crystals
E)network
A)metallic
B)ionic
C)molecular
D)liquid crystals
E)network

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements regarding the solid state is incorrect?
A)Solids are not very compressible.
B)Solids have rigid shapes and definite sizes.
C)If a material solidifies in a partially disordered state, it is an amorphous solid.
D)Substances which solidify in a very orderly manner form crystalline solids.
E)Glass is an example of a crystalline solid.
A)Solids are not very compressible.
B)Solids have rigid shapes and definite sizes.
C)If a material solidifies in a partially disordered state, it is an amorphous solid.
D)Substances which solidify in a very orderly manner form crystalline solids.
E)Glass is an example of a crystalline solid.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
Select the pair of substances which has the one with the higher equilibrium vapor pressure at a given temperature listed first.
A)H2O, H2S
B)CH3CH2OH, CH3OH
C)CH3COCH3, CH3CH2CH2OH
D)NH3, PH3
E)Br2, Cl2
A)H2O, H2S
B)CH3CH2OH, CH3OH
C)CH3COCH3, CH3CH2CH2OH
D)NH3, PH3
E)Br2, Cl2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
A solid substance has a high melting point, is hard, brittle, and conducts electricity when molten. This substance is probably a(n)________ solid.
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements regarding the liquid state is incorrect?
A)Detergents increase the surface tension of water, in order to help it stay in droplet form.
B)If ice were denser than liquid water, lakes would freeze from the bottom up.
C)In the summer, one would use a motor oil with a high viscosity rating to better lubricate the engine at high temperatures.
D)A liquid such as C15H32 would be expected to be more viscous than C6H14.
E)Water rises in a glass capillary tube due to the interactions between the water molecules and the glass.
A)Detergents increase the surface tension of water, in order to help it stay in droplet form.
B)If ice were denser than liquid water, lakes would freeze from the bottom up.
C)In the summer, one would use a motor oil with a high viscosity rating to better lubricate the engine at high temperatures.
D)A liquid such as C15H32 would be expected to be more viscous than C6H14.
E)Water rises in a glass capillary tube due to the interactions between the water molecules and the glass.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
A solid substance has a moderate melting point, is fairly soft, and is a nonconductor. This substance is probably a(n)________ solid.
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
A solid substance has a very high melting point, is very hard, and is a nonconductor whether molten or solid. This substance is probably a(n)________ solid.
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following statements regarding intermolecular forces is incorrect?
A)Hydrogen bonding is the strongest of the intermolecular forces for substances of similar molar mass.
B)London dispersion forces are present in all atoms and molecules.
C)Dipole-dipole forces increase with increasing molecular size.
D)Bonding forces are much stronger than intermolecular forces.
E)Hydrogen bonding is the reason for the abnormally high boiling point of water.
A)Hydrogen bonding is the strongest of the intermolecular forces for substances of similar molar mass.
B)London dispersion forces are present in all atoms and molecules.
C)Dipole-dipole forces increase with increasing molecular size.
D)Bonding forces are much stronger than intermolecular forces.
E)Hydrogen bonding is the reason for the abnormally high boiling point of water.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
Arrange the following substances in order of increasing boiling point: N2, H2, NH3, PH3
A)H2 < N2 < PH3 < NH3
B)N2 < H2 < PH3 < NH3
C)H2 < N2 < NH3 < PH3
D)N2 < PH3 < NH3 < H2
E)NH3 < PH3 < N2 < H2
A)H2 < N2 < PH3 < NH3
B)N2 < H2 < PH3 < NH3
C)H2 < N2 < NH3 < PH3
D)N2 < PH3 < NH3 < H2
E)NH3 < PH3 < N2 < H2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
Which choice correctly lists the intermolecular forces present in CH3NH2?
A)London forces only
B)dipole-dipole forces only
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)hydrogen bonding only
A)London forces only
B)dipole-dipole forces only
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)hydrogen bonding only

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
Select the pair of substances which has the one with the higher equilibrium vapor pressure at a given temperature listed first.
A)H2O, HCl
B)CH3OH, CH4
C)CH3CH2CH2OH, CH3COCH3
D)AsH3, PH3
E)Cl2, Br2
A)H2O, HCl
B)CH3OH, CH4
C)CH3CH2CH2OH, CH3COCH3
D)AsH3, PH3
E)Cl2, Br2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
A solid substance has a high melting point, is hard, and is a good conductor of both heat and electricity. It is also malleable and ductile. This substance is probably a(n)________ solid.
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
Which choice correctly lists the intermolecular forces present in CH4?
A)London forces only
B)London forces and dipole-dipole forces
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)London forces and hydrogen bonding
A)London forces only
B)London forces and dipole-dipole forces
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)London forces and hydrogen bonding

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
Arrange the following substances in order of increasing boiling point: CH3OH, CH4, CH3CH2OH, HOCH2CH2OH
A)CH3OH < CH4 < CH3CH2OH < HOCH2CH2OH
B)CH4 < CH3OH < CH3CH2OH < HOCH2CH2OH
C)CH4 < CH3OH < HOCH2CH2OH < CH3CH2OH
D)CH3OH < CH4 < HOCH2CH2OH < CH3CH2OH
E)HOCH2CH2OH < CH3CH2OH < CH3OH < CH4
A)CH3OH < CH4 < CH3CH2OH < HOCH2CH2OH
B)CH4 < CH3OH < CH3CH2OH < HOCH2CH2OH
C)CH4 < CH3OH < HOCH2CH2OH < CH3CH2OH
D)CH3OH < CH4 < HOCH2CH2OH < CH3CH2OH
E)HOCH2CH2OH < CH3CH2OH < CH3OH < CH4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements regarding the liquid state is incorrect?
A)Liquid water is denser than ice (solid water).
B)The viscosity of a liquid is related to its intermolecular forces-the stronger the forces, the higher the viscosity.
C)As temperature increases, so will the viscosity of a liquid.
D)Substances with strong intermolecular forces have high surface tension.
E)Detergents interfere with the attractive forces among water molecules to lower the surface tension of the water.
A)Liquid water is denser than ice (solid water).
B)The viscosity of a liquid is related to its intermolecular forces-the stronger the forces, the higher the viscosity.
C)As temperature increases, so will the viscosity of a liquid.
D)Substances with strong intermolecular forces have high surface tension.
E)Detergents interfere with the attractive forces among water molecules to lower the surface tension of the water.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck


