Exam 10: The Liquid and Solid States
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
Which substance has the highest melting point?
Free
(Multiple Choice)
4.8/5  (24)
(24)
Correct Answer:
D
Which of the molecules in the figure has hydrogen bonding in the pure liquid state? 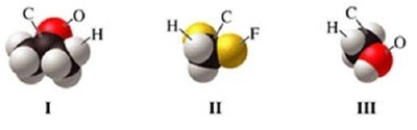
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
C
The physical properties of a substance influenced by the strength of intermolecular forces include all of the following except
Free
(Multiple Choice)
4.7/5  (47)
(47)
Correct Answer:
E
What phase transition is occurring between points B and C on the cooling curve? 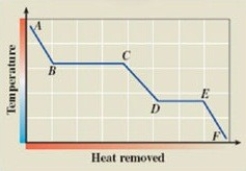
(Multiple Choice)
4.8/5  (45)
(45)
Explain how a pressure cooker works by filling in the blanks in the following statement: A pressure cooker allows the pressure inside the cooker to become ________ than atmospheric pressure, which ________ the boiling point of the water used to cook the food, thus cooking the food ________.
(Essay)
4.9/5  (40)
(40)
HCl boils at -85°C and F2 boils at -188°C. Which type of intermolecular force is responsible for the higher boiling point of HCl?
(Short Answer)
4.8/5  (38)
(38)
Calculate the amount of heat energy required to convert 27.5 g of water at 62.5°C to steam at 124.0°C. (Cwater = 4.184 J/g°C; Cice = 2.03 J/gºC; Csteam = 2.02 J/g°C; molar heat of fusion of ice = 6.01 × 103 J/mol; molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
(Multiple Choice)
4.9/5  (27)
(27)
The atmospheric pressure on Mount Everest is about 0.30 atm. From the vapor pressure curve of propane, it can be seen the boiling point of propane at this elevation is about ________. 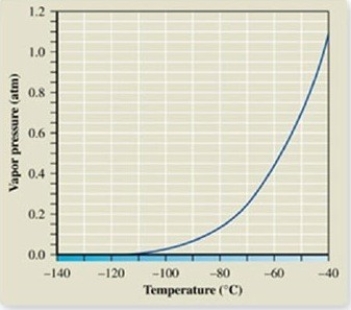
(Multiple Choice)
4.8/5  (37)
(37)
Select the pair of substances which has the one with the higher equilibrium vapor pressure at a given temperature listed first.
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
(Multiple Choice)
4.8/5  (33)
(33)
The physical properties of a substance influenced by the strength of intermolecular forces include all of the following except
(Multiple Choice)
4.8/5  (33)
(33)
A solid substance has a high melting point, is hard, brittle, and conducts electricity when molten. This substance is probably a(n)________ solid.
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the amount of heat energy required to evaporate 27.5 g of water at 100.0ºC. (molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
(Multiple Choice)
4.7/5  (36)
(36)
The forces that hold graphite, C(s), together in the solid state are
(Multiple Choice)
4.7/5  (49)
(49)
What is the boiling point in °C of acetone at 1.2 atm? 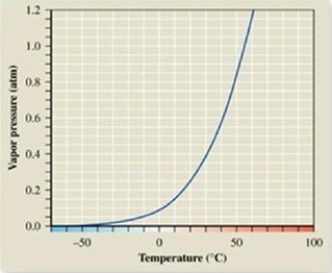
(Not Answered)
This question doesn't have any answer yet
Showing 1 - 20 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)