Deck 14: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 14: Chemical Kinetics
1
The reaction rate of a _____ reaction depends on the surface area of the more condensed phase.
heterogeneous
2
The _____ rate of a reaction is the reaction rate at any given point in time.
instantaneous
3
If there is contact between the constituent particles of two substances, the reaction rate will be zero.
False
4
Reaction rates generally decrease with time as reactant concentrations increase.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is used to calculate the overall reaction order?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the unit for reaction rate?
A) kJ/mol
B) C.m
C) kg.m2/s2
D) M/s
E) kJ/g
A) kJ/mol
B) C.m
C) kg.m2/s2
D) M/s
E) kJ/g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following best describes chemical kinetics?
A) It is the enthalpy change that occurs when a solid ionic compound is transformed into gaseous ions.
B) It is the process of converting one element to another.
C) It is a methodology that combines chemical reactions and stoichiometric calculations to determine the concentrations of substances present in a sample.
D) It is the study of reaction rates, the changes in the concentrations of reactants and products with time.
E) It is a complete description of the system at a given time, including its temperature and pressure, the amount of matter it contains, its chemical composition, and the physical state of the matter.
A) It is the enthalpy change that occurs when a solid ionic compound is transformed into gaseous ions.
B) It is the process of converting one element to another.
C) It is a methodology that combines chemical reactions and stoichiometric calculations to determine the concentrations of substances present in a sample.
D) It is the study of reaction rates, the changes in the concentrations of reactants and products with time.
E) It is a complete description of the system at a given time, including its temperature and pressure, the amount of matter it contains, its chemical composition, and the physical state of the matter.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
The _____ refers to the numbers that indicate the degree to which the reaction rate depends on the concentration of each reactant.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
Explain the effect of temperature on reaction rates with an example.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
The numerical value of the proportionality constant (k) changes as the reaction progresses under a given set of conditions.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
The reaction rate of virtually all reactions decreases with increasing temperature.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
A _____ is a substance that participates in a chemical reaction and increases the reaction rate without undergoing a net chemical change itself.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is true about the factors that affect reaction rates?
A) The reaction rate usually decreases as the concentration of the reactants increases.
B) The reaction rate of virtually all reactions decreases with increasing temperature.
C) The number of collisions between reactants per unit time is substantially reduced relative to the homogeneous case.
D) Catalysts do not influence the reaction and keep the reaction rate constant.
E) The reaction rates of most reactions increase rapidly with increasing solvent viscosity.
A) The reaction rate usually decreases as the concentration of the reactants increases.
B) The reaction rate of virtually all reactions decreases with increasing temperature.
C) The number of collisions between reactants per unit time is substantially reduced relative to the homogeneous case.
D) Catalysts do not influence the reaction and keep the reaction rate constant.
E) The reaction rates of most reactions increase rapidly with increasing solvent viscosity.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
The reaction rates of most reactions increase rapidly with increasing solvent viscosity.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
A _____ rate law expresses the reaction rate in terms of changes in the concentration of one or more reactants over a specific time interval.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate the reaction rate of SO2(g) in the following reaction, using the data provided in the following table.
A) 5.7
B) 2.5
C) 4.3
D) 1.3
E) 3.8
A) 5.7
B) 2.5
C) 4.3
D) 1.3
E) 3.8

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
The reaction rate of a heterogeneous reaction depends on the surface area of the more condensed phase.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
A(n)_____ rate law describes the reaction rate in terms of the initial concentration and the measured concentration of one or more reactants after a given amount of time.
A) homogeneous
B) degenerate
C) differential
D) heterogeneous
E) integrated
A) homogeneous
B) degenerate
C) differential
D) heterogeneous
E) integrated

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
Distinguish between differential and integrated rate laws.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
A graph of the concentration of any reactant as a function of time is a straight line with a slope of k.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
_____ reaction is one in which two smaller molecules, each called a monomer, combine to form a larger molecule.
A) Hydrolysis
B) Hybridization
C) Deformation
D) Fermentation
E) Dimerization
A) Hydrolysis
B) Hybridization
C) Deformation
D) Fermentation
E) Dimerization

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
Explain the zeroth-order reaction that takes place in the human liver during the oxidation of ethanol.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is an example of first-order reactions?
A) The hydrolysis of the anticancer drug cisplatin
B) The hydrolysis of aspirin
C) The oxidation of ethanol (from alcoholic beverages) to acetaldehyde in the liver
D) The reaction of t-butyl bromide with water to give t-butanol
E) The decomposition of on a platinum surface
A) The hydrolysis of the anticancer drug cisplatin
B) The hydrolysis of aspirin
C) The oxidation of ethanol (from alcoholic beverages) to acetaldehyde in the liver
D) The reaction of t-butyl bromide with water to give t-butanol
E) The decomposition of on a platinum surface

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
The following is an example of a first-order reaction involving the _____. 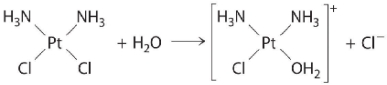
A) reaction of t-butyl bromide with water to give t-butanol
B) hydrolysis of the anticancer drug cisplatin
C) oxidation of ethanol to acetaldehyde in the liver
D) decomposition of N2O on a platinum surface
E) hydrolysis of aspirin

A) reaction of t-butyl bromide with water to give t-butanol
B) hydrolysis of the anticancer drug cisplatin
C) oxidation of ethanol to acetaldehyde in the liver
D) decomposition of N2O on a platinum surface
E) hydrolysis of aspirin

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is an example of first-order reactions?
A) The decomposition of N2O to N2 and O2
B) The reaction of t-butyl bromide with water to give t-butanol
C) The decomposition of HI to I2 and H2
D) A dimerization reaction
E) The oxidation of ethanol (from alcoholic beverages) to acetaldehyde in the liver
A) The decomposition of N2O to N2 and O2
B) The reaction of t-butyl bromide with water to give t-butanol
C) The decomposition of HI to I2 and H2
D) A dimerization reaction
E) The oxidation of ethanol (from alcoholic beverages) to acetaldehyde in the liver

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
In second-order reactions, doubling the concentration of the reactant quadruples the reaction rate.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
For a given number of atoms, isotopes with longer half-lives decay more rapidly.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
The rate of decay is the decrease in the number of a radioisotope's nuclei per unit time.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
A _____ order reaction is one whose rate is independent of concentration.
A) first
B) third
C) last
D) second
E) zeroth
A) first
B) third
C) last
D) second
E) zeroth

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
If a plot of reactant concentration versus time is not linear but a plot of the natural logarithm of reactant concentration versus time is linear, then the reaction is _____ order.
A) third
B) first
C) fourth
D) second
E) zeroth
A) third
B) first
C) fourth
D) second
E) zeroth

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is true of zeroth-order reactions?
A) The rate is dependent on the reactant concentration.
B) The differential rate law is rate = k.
C) The graph of the reactant concentration as a function of time is a straight line with slope of k.
D) It can be written in a form such that the exponent of the reactant in the rate law is 1.
E) The graph of the product concentration as a function of time is a straight line with slope of -k.
A) The rate is dependent on the reactant concentration.
B) The differential rate law is rate = k.
C) The graph of the reactant concentration as a function of time is a straight line with slope of k.
D) It can be written in a form such that the exponent of the reactant in the rate law is 1.
E) The graph of the product concentration as a function of time is a straight line with slope of -k.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
The following graph representing the concentration of the reactant versus time refers to _____-order reaction. 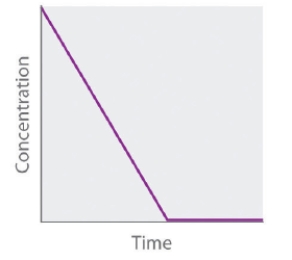
A) first
B) fourth
C) second
D) zeroth
E) third

A) first
B) fourth
C) second
D) zeroth
E) third

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
The hydrolysis of the anticancer drug cisplatin is an example of _____ order reaction.
A) first
B) second
C) last
D) third
E) zeroth
A) first
B) second
C) last
D) third
E) zeroth

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
For two or more reactions of the same order, the reaction with the smallest rate constant is the fastest.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
The following graph represents the straight-line plot to determine the rate constant of a _____-order reaction. 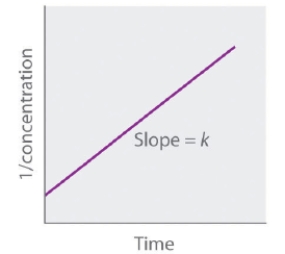
A) first
B) fourth
C) second
D) zeroth
E) third

A) first
B) fourth
C) second
D) zeroth
E) third

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
The sequence of reactions that occur at the molecular level during a reaction is called _____.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
A linear change in concentration with time is a clear indication of a _____ order reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
The half-life of a second-order reaction under a given set of reaction conditions is a constant.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
The rate of radioactive decay is dependent on temperature.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
A species in a reaction mechanism that does not appear in the balanced chemical equation for the overall reaction is known as the _____.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
The general rate law for a unimolecular elementary reaction (A → products) is rate = k[A].

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
The _____ of an elementary reaction is the number of molecules that collide during any step in a reaction mechanism.
A) molality
B) polarizability
C) molarity
D) molecularity
E) mass number
A) molality
B) polarizability
C) molarity
D) molecularity
E) mass number

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
_____ is a reaction mechanism in which one or more elementary reactions that contain a highly reactive species repeat again and again during the reaction process.
A) Transmutation
B) Reforming
C) Chain reaction
D) Galvanizing
E) Cracking
A) Transmutation
B) Reforming
C) Chain reaction
D) Galvanizing
E) Cracking

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
The half-life of a _____ reaction is independent of the concentration of the reactants.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
Explain chain reactions.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
In the following reaction, NO3 molecule does not appear in the balanced chemical equation for the overall reaction. This indicates that NO3 is a(n) _____ in the reaction.
A) intermediate
B) micelle
C) adduct
D) polymer
E) emulsion
A) intermediate
B) micelle
C) adduct
D) polymer
E) emulsion

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
The rate law for a termolecular elementary reaction of the form is _____.
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
The reaction rate will vary with concentration.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
The _____ of a reaction is the time required for the reactant concentration to decrease to one-half its initial value.
A) wavelength
B) frequency
C) overtone
D) amplitude
E) half-life
A) wavelength
B) frequency
C) overtone
D) amplitude
E) half-life

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
_____ reaction involves each of the complex series of reactions that take place in a stepwise fashion to convert reactants to products.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
A compound hydrolyzes in water with a rate constant of at pH 6.0 and . If a freshly prepared solution of the compound has a concentration of 0.086M, what is the percent completion of the reaction after 3 half-lives?
A) 63%
B) 87%
C) 94%
D) 79%
E) 56%
A) 63%
B) 87%
C) 94%
D) 79%
E) 56%

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
A compound hydrolyzes in water with a rate constant of at pH 6.0 and . If a freshly prepared solution of the compound has a concentration of 0.086 M, the concentration of the compound after 3 half-lives is _____ M.
A) 0.0120
B) 0.0110
C) 0.0106
D) 0.0108
E) 0.0104
A) 0.0120
B) 0.0110
C) 0.0106
D) 0.0108
E) 0.0104

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
_____ is the emission of a particle or a photon that results from the spontaneous decomposition of the unstable nucleus of an atom.
A) Photoelectric effect
B) Electron shielding
C) Blackbody radiation
D) Greenhouse effect
E) Radioactivity
A) Photoelectric effect
B) Electron shielding
C) Blackbody radiation
D) Greenhouse effect
E) Radioactivity

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
Explain the radioisotope dating techniques.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
Radicals are species that have one or more unpaired valence electrons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
The _____ of a sample of a radioactive substance is the decrease in the number of radioactive nuclei per unit time.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
A compound hydrolyzes in water with a rate constant of at pH 6.0 and . The half-life for this reaction is _____ min.
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
The fastest step in a reaction is the rate-determining step.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
The rate law for a reaction can be obtained from the overall balanced chemical equation alone.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
Which heterogeneous catalyst is used in steam reforming?
A) Ni
B) Fe
C) Cu
D) Pd
E) Rh
A) Ni
B) Fe
C) Cu
D) Pd
E) Rh

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
_____ is a constant in the Arrhenius equation which converts concentrations to collisions per second.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
What does indicate in the Arrhenius equation?
A) Ideal gas constant
B) Avogadro's number
C) Boltzmann's constant
D) Atomic mass unit
E) Faraday's constant
A) Ideal gas constant
B) Avogadro's number
C) Boltzmann's constant
D) Atomic mass unit
E) Faraday's constant

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
For two similar reactions under comparable conditions, the reaction with the greatest will occur more rapidly.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
Enzymes are catalysts that occur naturally in living organisms.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
The fraction of orientations that result in a reaction is called the _____.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
A catalyst affects but not Ea.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following refers to Arrhenius equation?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is a commercially important reaction that employs heterogeneous catalysts?
A) Hydroperoxide process
B) Adiponitrile process
C) Olefin polymerization
D) Methanol synthesis
E) Hydroformylation
A) Hydroperoxide process
B) Adiponitrile process
C) Olefin polymerization
D) Methanol synthesis
E) Hydroformylation

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
_____ is a substance that participates in a reaction and causes it to occur more rapidly but that can be recovered unchanged at the end of the reaction and reused.
A) Poison
B) Catalyst
C) Polymer
D) Adduct
E) Allotrope
A) Poison
B) Catalyst
C) Polymer
D) Adduct
E) Allotrope

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following feature is true about catalysts?
A) Catalysts appear in the overall stoichiometry of the reaction being catalyzed.
B) The reaction rate of a catalyzed reaction is slower than the reaction rate of the uncatalyzed reaction at the same temperature.
C) Catalysts increase the reaction rate of a chemical reaction by being consumed in the process.
D) The net change in energy that results from a reaction is affected by the presence of a catalyst.
E) Catalysts increase the reaction rates of both the forward and the reverse reactions by the same amount.
A) Catalysts appear in the overall stoichiometry of the reaction being catalyzed.
B) The reaction rate of a catalyzed reaction is slower than the reaction rate of the uncatalyzed reaction at the same temperature.
C) Catalysts increase the reaction rate of a chemical reaction by being consumed in the process.
D) The net change in energy that results from a reaction is affected by the presence of a catalyst.
E) Catalysts increase the reaction rates of both the forward and the reverse reactions by the same amount.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
Explain activation energy with an example.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is a commercially important reaction that employs homogeneous catalysts?
A) Ostwald process
B) Hydroformylation
C) Haber process
D) Methanol synthesis
E) Catalytic hydrogenation
A) Ostwald process
B) Hydroformylation
C) Haber process
D) Methanol synthesis
E) Catalytic hydrogenation

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
The arrangement of atoms that first forms when molecules are able to overcome the activation energy and react is called _____.
A) an activated complex
B) the smectic phase
C) a bilayer
D) the cholesteric phase
E) a micelle
A) an activated complex
B) the smectic phase
C) a bilayer
D) the cholesteric phase
E) a micelle

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
According to the _____, a chemical reaction can occur only when the reactant molecules, atoms, or ions collide with more than a certain amount of kinetic energy and in the proper orientation.
A) Lewis bonding model
B) Bohr's model
C) Valence-shell electron-pair repulsion model
D) collision model
E) Rutherford's model
A) Lewis bonding model
B) Bohr's model
C) Valence-shell electron-pair repulsion model
D) collision model
E) Rutherford's model

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
Differentiate between heterogeneous and homogeneous catalysts.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
The activated complex is a reaction intermediate.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
_____ are enzymes that are able to cleave the amide bonds that hold amino acids together in proteins.
A) Poisons
B) Substrates
C) Adducts
D) Micelles
E) Proteases
A) Poisons
B) Substrates
C) Adducts
D) Micelles
E) Proteases

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
A steric factor value of 0 indicates that all orientations result in reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
High-density polyethylene and polypropylene are produced by heterogeneous catalysis

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck


