Exam 14: Chemical Kinetics
Exam 1: Introduction to Chemistry96 Questions
Exam 2: Molecules, Ions, and Chemical Formulas110 Questions
Exam 3: Chemical Reactions88 Questions
Exam 4: Reactions in Aqueous Solution95 Questions
Exam 5: Energy Changes in Chemical Reactions93 Questions
Exam 6: The Structure of Atoms110 Questions
Exam 7: The Periodic Table and Periodic Trends97 Questions
Exam 8: Ionic Versus Covalent Bonding88 Questions
Exam 9: Molecular Geometry and Covalent Bonding Models89 Questions
Exam 10: Gases84 Questions
Exam 11: Liquids99 Questions
Exam 12: Solids97 Questions
Exam 13: Solutions96 Questions
Exam 14: Chemical Kinetics85 Questions
Exam 15: Chemical Equilibrium73 Questions
Exam 16: Aqueous Acidbase Equilibriums97 Questions
Exam 17: Solubility and Complexation Equilibriums85 Questions
Exam 18: Chemical Thermodynamics108 Questions
Exam 19: Electrochemistry95 Questions
Exam 20: Nuclear Chemistry95 Questions
Exam 21: Periodic Trends and the S-Block Elements93 Questions
Exam 22: The P-Block Elements94 Questions
Exam 23: The D-Block Elements95 Questions
Exam 24: Organic Compounds111 Questions
Select questions type
The half-life of a second-order reaction under a given set of reaction conditions is a constant.
Free
(True/False)
4.9/5  (32)
(32)
Correct Answer:
False
A _____ order reaction is one whose rate is independent of concentration.
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
E
Calculate the reaction rate of SO2(g) in the following reaction, using the data provided in the following table.
Time(s) () () () 200 0.0235 0.0520 0.0100 520 0.0146 0.0410 0.0208
Free
(Multiple Choice)
4.8/5  (17)
(17)
Correct Answer:
D
Radicals are species that have one or more unpaired valence electrons.
(True/False)
4.7/5  (35)
(35)
The following graph represents the straight-line plot to determine the rate constant of a _____-order reaction. 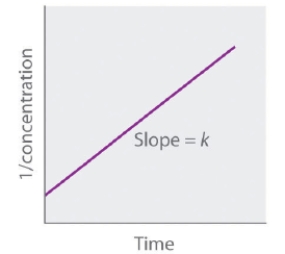
(Multiple Choice)
4.8/5  (30)
(30)
A graph of the concentration of any reactant as a function of time is a straight line with a slope of k.
(True/False)
4.9/5  (34)
(34)
The general rate law for a unimolecular elementary reaction (A → products) is rate = k[A].
(True/False)
4.8/5  (31)
(31)
A(n)_____ rate law describes the reaction rate in terms of the initial concentration and the measured concentration of one or more reactants after a given amount of time.
(Multiple Choice)
4.8/5  (31)
(31)
The rate law for a reaction can be obtained from the overall balanced chemical equation alone.
(True/False)
4.7/5  (39)
(39)
Irreversible inhibitors are therefore the equivalent of _____ in heterogeneous catalysis.
(Short Answer)
4.8/5  (36)
(36)
The following is an example of a first-order reaction involving the _____. 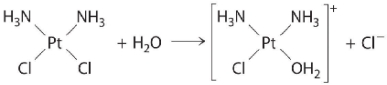
(Multiple Choice)
4.9/5  (33)
(33)
Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction.
(True/False)
4.9/5  (33)
(33)
The reaction rate of a heterogeneous reaction depends on the surface area of the more condensed phase.
(True/False)
4.8/5  (25)
(25)
Reaction rates generally decrease with time as reactant concentrations increase.
(True/False)
4.9/5  (36)
(36)
A compound hydrolyzes in water with a rate constant of at pH 6.0 and . The half-life for this reaction is _____ min.
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)