Deck 11: Liquids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 11: Liquids
1
Explain why many nonpolar molecules, such as benzene and hexane, are liquids at room temperature.
Fritz London explained that the temporary fluctuations in the electron distributions within atoms and nonpolar molecules could result in the formation of short-lived instantaneous dipole moments. The instantaneous dipole moment on one atom can interact with the electrons in an adjacent atom, pulling them toward the positive end of the instantaneous dipole or repelling them from the negative end. The net effect is that the first atom causes the temporary formation of a dipole, called an induced dipole, in the second. Interactions between these temporary dipoles cause atoms to be attracted to one another. These attractive forces are called London dispersion forces. Hence nonpolar substances are liquids at room temperature.
2
Which of the following statements is true regarding the properties of liquids?
A) Liquids are much denser than solids.
B) Liquids can be readily compressed.
C) The density of water varies significantly over a narrow temperature range.
D) Liquids can adopt the volume of their containers.
E) The mean free path in liquids is very short.
A) Liquids are much denser than solids.
B) Liquids can be readily compressed.
C) The density of water varies significantly over a narrow temperature range.
D) Liquids can adopt the volume of their containers.
E) The mean free path in liquids is very short.
The mean free path in liquids is very short.
3
The compounds, potassium cyanide, diethylether, and cyclobutane when arranged in an increasing order of their boiling points, will follow the order:
A) potassium cyanide ˂ diethyl ether ˂ cyclobutane.
B) potassium cyanide ˂ cyclobutane ˂ diethyl ether.
C) diethyl ether ˂ cyclobutane ˂ potassium cyanide.
D) cyclobutane ˂ potassium cyanide ˂ diethyl ether.
E) cyclobutane ˂ diethyl ether ˂ potassium cyanide.
A) potassium cyanide ˂ diethyl ether ˂ cyclobutane.
B) potassium cyanide ˂ cyclobutane ˂ diethyl ether.
C) diethyl ether ˂ cyclobutane ˂ potassium cyanide.
D) cyclobutane ˂ potassium cyanide ˂ diethyl ether.
E) cyclobutane ˂ diethyl ether ˂ potassium cyanide.
cyclobutane ˂ diethyl ether ˂ potassium cyanide.
4
Why do liquids adopt the shape, but not the volume of their containers?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
The arrangement of molecules in a _____ is completely random.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
The intermolecular interactions that result between molecules with net dipole moments are called _____.
A) instantaneous dipole moments
B) dipole-dipole interactions
C) dipole-ion interactions
D) London dispersion forces
E) hydrogen bonds
A) instantaneous dipole moments
B) dipole-dipole interactions
C) dipole-ion interactions
D) London dispersion forces
E) hydrogen bonds

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
HCl is a liquid at room temperature and 1 atm pressure.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
London dispersion forces get stronger with increasing molecular size for similar substances.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
The density of a liquid is commonly measured in units of _____.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
The rate of diffusion in liquids is faster than that in gases.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Why is ice less dense than liquid water?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
A three fold increase in the distance between two dipoles of adjacent molecules:
A) decreases the strength of interactions by 64 fold.
B) increases the strength of interaction by 26.
C) has no influence on the strength of interactions between the dipoles.
D) decreases the strength of interactions by 729 fold.
E) decreases the strength of interactions by 216 fold.
A) decreases the strength of interactions by 64 fold.
B) increases the strength of interaction by 26.
C) has no influence on the strength of interactions between the dipoles.
D) decreases the strength of interactions by 729 fold.
E) decreases the strength of interactions by 216 fold.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is true of the molecular order of liquids?
A) The arrangement of molecules in liquids is completely random.
B) The molecules have a lower kinetic energy compared to the molecules in a solid.
C) The molecules in a liquid can move freely within the system without colliding with each other.
D) The molecules exhibit short-range order because of strong intermolecular attractive forces.
E) The molecules in a liquid are arranged in a repeating three-dimensional array.
A) The arrangement of molecules in liquids is completely random.
B) The molecules have a lower kinetic energy compared to the molecules in a solid.
C) The molecules in a liquid can move freely within the system without colliding with each other.
D) The molecules exhibit short-range order because of strong intermolecular attractive forces.
E) The molecules in a liquid are arranged in a repeating three-dimensional array.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following compounds has the highest boiling point?
A) Cyclobutane
B) Acetone
C) Dimethyl sulfoxide
D) Diethylether
E) Hydrogen cyanide
A) Cyclobutane
B) Acetone
C) Dimethyl sulfoxide
D) Diethylether
E) Hydrogen cyanide

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
The ease of deformation of the electron distribution in an atom or molecule is called its _____.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
The kinetic energy of water molecules depends on their temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
Intramolecular forces hold molecules together in a liquid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
A short-lived dipole moment that is created in atoms and nonpolar molecules adjacent to atoms or molecules with an instantaneous dipole moment is called a(n) _____.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
The arrangement of molecules in a liquid is in a repeating three-dimensional array.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
A(n) _____ is a strong dipole-dipole interaction that results when hydrogen is bonded to electronegative elements such as oxygen, nitrogen and fluorine.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
The upper surface of a liquid in a tube is called the _____.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
How do the relative strengths of cohesive and adhesive forces determine the level to which a liquid rises in a capillary tube?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Viscosity of a liquid is defined as:
A) the ratio of the liquid's volume to its mass.
B) the energy required to increase the outer area of a liquid by a specific amount.
C) the mass of a liquid per unit volume.
D) the resistance of a liquid to flow.
E) the tendency of the liquid to rise against gravity.
A) the ratio of the liquid's volume to its mass.
B) the energy required to increase the outer area of a liquid by a specific amount.
C) the mass of a liquid per unit volume.
D) the resistance of a liquid to flow.
E) the tendency of the liquid to rise against gravity.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a volatile liquid?
A) Glycerine
B) Acetone
C) Ethylene glycol
D) Water at 60oC
E) Mercury
A) Glycerine
B) Acetone
C) Ethylene glycol
D) Water at 60oC
E) Mercury

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
The surface tension of a liquid is measured in units of _____.
A) g/cm3
B) J/˚C
C) Pa·s
D) J/m2
E) J/cm2
A) g/cm3
B) J/˚C
C) Pa·s
D) J/m2
E) J/cm2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Explain with an example, the practical importance of controlling the viscosity of liquids.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following has the lowest vapor pressure?
A) Ethylene glycol
B) Acetone
C) Water at 60oC
D) Diethyl ether
E) Gasoline
A) Ethylene glycol
B) Acetone
C) Water at 60oC
D) Diethyl ether
E) Gasoline

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
The intermolecular forces that hold a liquid together are called _____.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
Condensation is a physical process by which the atoms or molecules:
A) in the liquid phase enter the gas phase.
B) in the vapor phase enter the solid phase.
C) in the vapor phase enter the liquid phase.
D) in the solid phase enter the liquid phase.
E) in the solid phase enter the gas phase.
A) in the liquid phase enter the gas phase.
B) in the vapor phase enter the solid phase.
C) in the vapor phase enter the liquid phase.
D) in the solid phase enter the liquid phase.
E) in the solid phase enter the gas phase.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
The rate of condensation depends only on the surface area of the liquid and is essentially constant.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements is true of the capillary action of liquids?
A) The capillary action of a liquid entirely depends on the strength of the adhesive forces.
B) If the adhesive forces are weaker than the cohesive forces, then the liquid in the capillary rises to the level where the downward force of gravity exactly balances this upward force.
C) If the cohesive forces are stronger than the adhesive forces, the liquid in the tube will have a convex meniscus.
D) If the cohesive forces are weaker than the adhesive forces, the liquid pulls itself down into the capillary below the surface of the bulk liquid to minimize contact with the glass.
E) If the cohesive forces are weaker than the adhesive forces, the liquid in the tube will have a convex meniscus.
A) The capillary action of a liquid entirely depends on the strength of the adhesive forces.
B) If the adhesive forces are weaker than the cohesive forces, then the liquid in the capillary rises to the level where the downward force of gravity exactly balances this upward force.
C) If the cohesive forces are stronger than the adhesive forces, the liquid in the tube will have a convex meniscus.
D) If the cohesive forces are weaker than the adhesive forces, the liquid pulls itself down into the capillary below the surface of the bulk liquid to minimize contact with the glass.
E) If the cohesive forces are weaker than the adhesive forces, the liquid in the tube will have a convex meniscus.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
A sphere has the smallest possible surface area for a given volume.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
The molecules that are present only at the surface of a liquid can undergo evaporation.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following polar liquids will exhibit a high capillary action?
A) mercury
B) acetone
C) olive oil
D) glycerol
E) liquid methane
A) mercury
B) acetone
C) olive oil
D) glycerol
E) liquid methane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
The equilibrium vapor pressure of a liquid strongly depends on the amount of liquid present.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
The physical process by which atoms or molecules in the liquid phase enter the gas phase is known as _____.
A) sublimation
B) condensation
C) deposition
D) fusion
E) vaporization
A) sublimation
B) condensation
C) deposition
D) fusion
E) vaporization

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following has the highest viscosity?
A) Ethanol
B) Chloroform
C) Castor oil
D) Milk
E) Gasoline
A) Ethanol
B) Chloroform
C) Castor oil
D) Milk
E) Gasoline

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
_____ is a unique property of water that helps in the transportation of fluids and nutrients, from the roots to the top of trees through the trunks.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
Viscosity decreases with the increasing molecular size.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Water has a convex meniscus.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
A change of state that occurs when any of the three forms of matter is converted to either of the other two is called a(n) _____.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
The pressure exerted by a vapor in dynamic equilibrium with its liquid is the _____ of the liquid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
The enthalpy change that accompanies the conversion of a solid directly to a gas is called enthalpy of _____.
A) combustion
B) sublimation
C) fusion
D) vaporization
E) solution
A) combustion
B) sublimation
C) fusion
D) vaporization
E) solution

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
In the Clausius-Clapeyron equation, ln P = represents:
A) heat of vaporization
B) vaporization
C) absolute temperature
D) universal gas constant
E) Clapeyron constant
A) heat of vaporization
B) vaporization
C) absolute temperature
D) universal gas constant
E) Clapeyron constant

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
The enthalpy of vaporization of a given substance is greater than its enthalpy of fusion.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following physical processes is a reverse of the process of sublimation?
A) Condensation
B) Deposition
C) Fusion
D) Freezing
E) Vaporization
A) Condensation
B) Deposition
C) Fusion
D) Freezing
E) Vaporization

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
The vapor pressure of methanol at 50.0˚C and 65.0˚C is 400.0 and 760.0 torr respectively. The enthalpy of vaporization of methanol will be _____ kJ/mol.
A) 3.89
B) 391
C) 38.8
D) 107
E) 65.0
A) 3.89
B) 391
C) 38.8
D) 107
E) 65.0

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
A supercritical fluid has a density which is almost the same as the density of the gas phase.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
Phase changes are always accompanied by a change in the temperature of the system.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
A(n) _____ refers to an unstable liquid at a temperature and pressure at which it should be a gas.
A) ionic liquid
B) superheated liquid
C) supercooled liquid
D) supercritical fluid
E) viscous liquid
A) ionic liquid
B) superheated liquid
C) supercooled liquid
D) supercritical fluid
E) viscous liquid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
The physical process of conversion of a solid to a liquid is known as _____.
A) sublimation
B) condensation
C) evaporation
D) fusion
E) deposition
A) sublimation
B) condensation
C) evaporation
D) fusion
E) deposition

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
The _____ refers to the pressure created over a liquid by the molecules of a liquid substance that have enough kinetic energy to escape to the vapor phase.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
A metastable liquid phase that exists below the normal melting point of a substance is known as _____.
A) Ionic liquid
B) Superheated liquid
C) Supercooled liquid
D) Supercritical fluid
E) Superheated steam
A) Ionic liquid
B) Superheated liquid
C) Supercooled liquid
D) Supercritical fluid
E) Superheated steam

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
A(n) _____ is a solid sample of a substance that can be added to a supercooled liquid or a supersaturated solution to help induce crystallization.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
Explain why there is no change in temperature during a phase change.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
What is dynamic equilibrium? Why there is no equilibrium established in the case of a liquid in an open container?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is an endothermic process?
A) Conversion of supercooled liquid to glass.
B) Liquefaction of natural gas.
C) Formation of frost on leaves.
D) Loss of snow due to sunshine activity.
E) Corrosion of metals.
A) Conversion of supercooled liquid to glass.
B) Liquefaction of natural gas.
C) Formation of frost on leaves.
D) Loss of snow due to sunshine activity.
E) Corrosion of metals.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
The vapor pressure of liquid water at 58.0˚C and 73.0˚C is 136.1 and 265.7 torr respectively. The vapor pressure of liquid water at 69.0˚C is _____ torr.
A) 137
B) 425
C) 223
D) 265
E) 416
A) 137
B) 425
C) 223
D) 265
E) 416

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
The transition from a solid to a gas is exothermic, as it releases energy.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following processes best exemplifies the process of sublimation?
A) Coal liquefaction
B) Melting of snow
C) The process of crystallization
D) The process of distillation
E) Iodine producing fumes on heating
A) Coal liquefaction
B) Melting of snow
C) The process of crystallization
D) The process of distillation
E) Iodine producing fumes on heating

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
Molten salts are good conductors of electricity.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
_____ are substances that are liquids at room temperature and pressure due to their disorganized structure that results in a relatively low melting point.
A) Superheated liquids
B) Supercritical fluids
C) Ionic liquids
D) Molten salts
E) Supercooled liquids
A) Superheated liquids
B) Supercritical fluids
C) Ionic liquids
D) Molten salts
E) Supercooled liquids

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following lines in the given phase diagram, corresponds to a line along which the solid and liquid phases are in equilibrium?
A) The line that connects points B and D.
B) The line that connects points C and B.
C) The line that connects points A and B.
D) The line that connects points A and C.
E) The line that connects points A and D.
A) The line that connects points B and D.
B) The line that connects points C and B.
C) The line that connects points A and B.
D) The line that connects points A and C.
E) The line that connects points A and D.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following lines in the given phase diagram, represents the vapor pressure curve of the solid phase?
A) The line that connects points B and D.
B) The line that connects points C and B.
C) The line that connects points A and B.
D) The line that connects points A and C.
E) The line that connects points A and D.
A) The line that connects points B and D.
B) The line that connects points C and B.
C) The line that connects points A and B.
D) The line that connects points A and C.
E) The line that connects points A and D.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
The line that connects points A and B in the given phase diagram is:
A) is the vapor pressure curve of the solid phase.
B) shows the melting point variation of a solid with pressure.
C) is the vapor pressure curve of the liquid phase.
D) is the vapor pressure curve of the gas phase.
E) shows the boiling point variation of a liquid with pressure.
A) is the vapor pressure curve of the solid phase.
B) shows the melting point variation of a solid with pressure.
C) is the vapor pressure curve of the liquid phase.
D) is the vapor pressure curve of the gas phase.
E) shows the boiling point variation of a liquid with pressure.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
In a phase diagram, the region that corresponds to a combination of high pressure and low temperature favors _____ phase.
A) gas
B) supercritical fluid
C) supercooled liquid
D) solid
E) liquid
A) gas
B) supercritical fluid
C) supercooled liquid
D) solid
E) liquid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
When solid carbon dioxide is heated, it undergoes sublimation to form a gas.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
A gas phase is favored at high pressure and low temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
The combination of temperature and pressure at which all three forms of matter can exist simultaneously is called the critical point.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
The combination of the critical temperature and critical pressure of a substance is called its _____.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
The point in a phase diagram that represents the only combination of temperature and pressure at which all three forms of matter are in equilibrium is called the _____ point.
A) equilibrium
B) critical
C) significant
D) triple
E) dominant
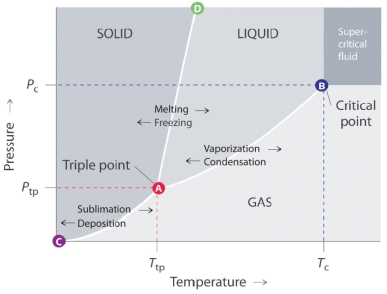
A) equilibrium
B) critical
C) significant
D) triple
E) dominant


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
A salt when heated to its melting point produces a(n) _____.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
What are ionic liquids? List any two applications of ionic liquids.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following substances has the highest critical temperature?
A) Ammonia
B) Mercury
C) Carbon dioxide
D) Water
E) Ethanol
A) Ammonia
B) Mercury
C) Carbon dioxide
D) Water
E) Ethanol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
The "_____" point of a substance is a combination of the highest temperature at which it can exist as a liquid and the minimum pressure needed to liquefy it at that temperature.
A) significant
B) dominant
C) superficial
D) critical
E) specific
A) significant
B) dominant
C) superficial
D) critical
E) specific

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
The single, dense fluid phase that exists above the critical temperature of a substance is called a(n) _____ fluid.
A) ionic
B) superheated
C) molten
D) supercritical
E) supercooled
A) ionic
B) superheated
C) molten
D) supercritical
E) supercooled

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
List out the general features of a phase diagram. 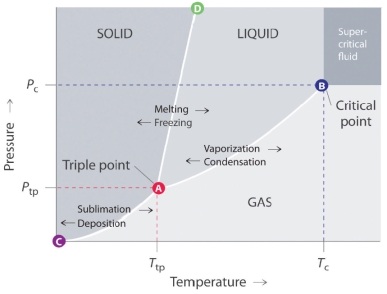


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
A(n) _____ is a graphic summary of the physical state of a substance as a function of temperature and pressure in a closed system.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
Ionic liquids have low melting points.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
The region that corresponds to a combination of high temperature and low pressure in a phase diagram favours _____ phase.
A) supercritical fluid
B) gas
C) supercooled liquid
D) solid
E) liquid
A) supercritical fluid
B) gas
C) supercooled liquid
D) solid
E) liquid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


