Exam 11: Liquids
List out the general features of a phase diagram. 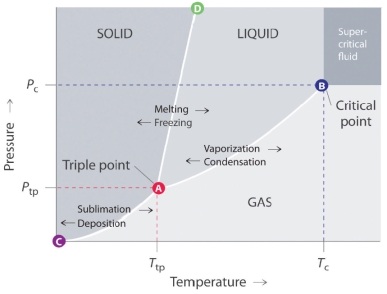
A typical phase diagram consists of discrete regions that represent the different phases exhibited by a substance. Each region corresponds to the range of combinations of temperature and pressure over which that phase is stable.
The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium.
a. The line that connects points A and D separates the solid and liquid phases and shows how the melting point of a solid varies with pressure.
b. The line that connects points A and B is the vapor pressure curve of the liquid. It ends at the critical point, beyond which the substance exists as a supercritical fluid.
c. The line that connects points A and C is the vapor pressure curve of the solid phase.
The point A represents the triple point, which is the only combination of temperature and pressure at which all three forms of matter are in equilibrium, and hence can exist simultaneously.
What are ionic liquids? List any two applications of ionic liquids.
Ionic liquids are ionic substances that are liquid at room temperature and pressure, and consist of small, symmetrical anions, combined with larger, asymmetrical organic cations that prevent the formation of a highly organized structure.
Examples: tetrapentylammonium bromide, 1-butyl-3-methylimidazolium hexafluorophosphate
Students' answers might vary for applications.
Ionic liquids can be used to extract specific molecules from a solution. They can also be used to produce commercially important organic compounds such as, Styrofoam and Plexiglas.
Which of the following substances has the highest critical temperature?
B
The transition from a solid to a gas is exothermic, as it releases energy.
Why do liquids adopt the shape, but not the volume of their containers?
The enthalpy change that accompanies the conversion of a solid directly to a gas is called enthalpy of _____.
When solid carbon dioxide is heated, it undergoes sublimation to form a gas.
Answer the questions based on the phase diagram given below
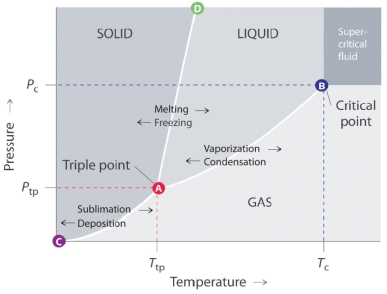 -The line that connects points A and B in the given phase diagram is:
-The line that connects points A and B in the given phase diagram is:
In a phase diagram, the region that corresponds to a combination of high pressure and low temperature favors _____ phase.
The surface tension of a liquid is measured in units of _____.
An arrangement of molecules in which their properties depend on the direction they are measured is described as _____.
Which of the following physical processes is a reverse of the process of sublimation?
In which of the following orientations of liquid crystals, the molecules are directionally oriented and stacked in a helical pattern, with each layer rotated at a slight angle to the ones above and below it?
In the _____ phase, the liquid crystal molecules are relatively more opaque compared to the other orientations.
The vapor pressure of methanol at 50.0˚C and 65.0˚C is 400.0 and 760.0 torr respectively. The enthalpy of vaporization of methanol will be _____ kJ/mol.
What is dynamic equilibrium? Why there is no equilibrium established in the case of a liquid in an open container?
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)