Deck 40: Introduction to Quantum Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/48
Play
Full screen (f)
Deck 40: Introduction to Quantum Physics
1
A solid state pulsed laser has an energy of 400 mJ per pulse. If its wavelength is 1.06 × 10−6 m, how many photons are in each pulse?
A) 2 × 1025
B) 2 × 1021
C) 3 × 1018
D) 6 × 1038
E) 2 × 1018
A) 2 × 1025
B) 2 × 1021
C) 3 × 1018
D) 6 × 1038
E) 2 × 1018
2 × 1018
2
A helium-neon laser emits red light having a wavelength of 6.4 × 10−7 m and a power of 0.5 mW. How many photons are emitted each second?
A) 6.4 × 1038
B) 1.6 × 1021
C) 3.2 × 1025
D) 2.6 × 1018
E) 1.6 × 1015
A) 6.4 × 1038
B) 1.6 × 1021
C) 3.2 × 1025
D) 2.6 × 1018
E) 1.6 × 1015
1.6 × 1015
3
An electron is accelerated through a potential difference of 25000 V. What is the de Broglie wavelength of the electron (in m)?
A) 5.9 × 10−12
B) 6.8 × 10−12
C) 6.5 × 10−12
D) 7.8 × 10−12
E) 5.5 × 10−12
A) 5.9 × 10−12
B) 6.8 × 10−12
C) 6.5 × 10−12
D) 7.8 × 10−12
E) 5.5 × 10−12
7.8 × 10−12
4
A photon whose energy is 8.00 × 10−15 J is scattered off an electron at a 90° angle. What is the wavelength of the scattered wave?
A) 2.73 × 10−11 m
B) 2.25 × 10−11 m
C) 2.50 × 10−11 m
D) 2.40 × 10−12 m
E) 2.48 × 10−11 m
A) 2.73 × 10−11 m
B) 2.25 × 10−11 m
C) 2.50 × 10−11 m
D) 2.40 × 10−12 m
E) 2.48 × 10−11 m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
5
What is the maximum kinetic energy (in eV) of a photoelectron when a surface, whose work function is 5.0 eV, is illuminated by photons whose wavelength is 400 nm?
A) 3.1
B) −1.9
C) 1.9
D) 0
E) 1.2
A) 3.1
B) −1.9
C) 1.9
D) 0
E) 1.2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
6
Your temperature is 98.6°F. Assuming your skin is a perfect radiator (ε = 1), determine the wavelength corresponding to the largest intensity (in μm).
A) 8.0
B) 9.3
C) 3.0
D) 5.7
E) 29.4
A) 8.0
B) 9.3
C) 3.0
D) 5.7
E) 29.4

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
7
A neutron has a mass of 1.67 × 10−27 kg. Its de Broglie wavelength is 1.4 × 10−10 m. What is its kinetic energy (in eV)?
A) 4
B) 0.4
C) 0.04
D) 40
E) 0.08
A) 4
B) 0.4
C) 0.04
D) 40
E) 0.08

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
8
A photon whose wavelength is = 5.0 × 10−11 m is scattered straight backward. What is the wavelength of the scattered wave?
A) 5.0 × 10−11 m
B) 4.5 × 10−11 m
C) 5.5 × 10−11 m
D) 6.0 × 10−11 m
E) 6.5 × 10−11 m
A) 5.0 × 10−11 m
B) 4.5 × 10−11 m
C) 5.5 × 10−11 m
D) 6.0 × 10−11 m
E) 6.5 × 10−11 m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
9
A photon collides with an electron. After the collision the wavelength of the scattered wave at a scattering angle greater than 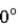 is
is
A) greater than or equal to the initial wavelength.
B) equal to the initial wavelength.
C) less than or equal to the initial wavelength.
D) greater than the initial wavelength.
E) less or greater depending on the scattering angle.
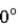 is
isA) greater than or equal to the initial wavelength.
B) equal to the initial wavelength.
C) less than or equal to the initial wavelength.
D) greater than the initial wavelength.
E) less or greater depending on the scattering angle.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
10
How much energy is in a 63 kHz photon of AM-radiation?
A) 1.0 × 10−38 J
B) 6.6 × 10−30 J
C) 4.2 × 10−29 J
D) 3.1 × 10−30 J
E) 13 × 10−29 J
A) 1.0 × 10−38 J
B) 6.6 × 10−30 J
C) 4.2 × 10−29 J
D) 3.1 × 10−30 J
E) 13 × 10−29 J

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
11
The light intensity incident on a metallic surface with a work function of 3 eV produces photoelectrons with a maximum kinetic energy of 2 eV. The frequency of the light is doubled. Determine the maximum kinetic energy (in eV).
A) 3
B) 2
C)
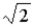
D) 4
E) 7
A) 3
B) 2
C)
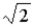
D) 4
E) 7

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
12
A stopping potential of 3.2 V is needed for radiation whose wavelength is 200 nm. What is the work function (in eV) of the material?
A) 4.0
B) 3.0
C) 5.0
D) 6.0
E) 2.0
A) 4.0
B) 3.0
C) 5.0
D) 6.0
E) 2.0

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
13
What is the maximum kinetic energy (in eV) of a photoelectron emitted from a surface whose work function is 5.0 eV when illuminated by a light whose wavelength is 200 nm?
A) 1.9
B) 1.2
C) 3.1
D) zero
E) 6.2
A) 1.9
B) 1.2
C) 3.1
D) zero
E) 6.2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
14
Assume electrons are accelerated through a potential difference of 25000 V inside a TV picture tube. What is the minimum wavelength that could be produced when the electrons strike the phosphor? (1 Å = 10−10 m)
A) 0.50 Å
B) 1.0 Å
C) 10 Å
D) 100 Å
E) 0.25 Å
A) 0.50 Å
B) 1.0 Å
C) 10 Å
D) 100 Å
E) 0.25 Å

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
15
A neutron has a mass of 1.67 × 10−27 kg. The de Broglie wavelength is 1.4 × 10−10 m. How fast is the neutron going? (in m/s)
A) 3.4 × 103
B) 2.8 × 103
C) 3.9 × 103
D) 2.6 × 103
E) 1.7 × 103
A) 3.4 × 103
B) 2.8 × 103
C) 3.9 × 103
D) 2.6 × 103
E) 1.7 × 103

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
16
A neutron has a mass of 1.67 × 10−27 kg. Its de Broglie wavelength is 1.4 × 10−10 m. What temperature would it correspond to if we had a monatomic gas having the same average kinetic energy (in °C)?
A) 273
B) 25
C) 36
D) 309
E) 51
A) 273
B) 25
C) 36
D) 309
E) 51

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
17
What is the maximum velocity (in km/s) of a photoelectron emitted from a surface whose work function is 5.0 eV when illuminated by a light whose wavelength is 200 nm?
A) 460
B) 650
C) 420
D) 550
E) 1 480
A) 460
B) 650
C) 420
D) 550
E) 1 480

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
18
The threshold wavelength for photoelectric emission of a particular substance is 500 nm. What is the work function (in eV)?
A) 4.2
B) 4.0 × 10−19
C) 4.0 × 10−10
D) 2.5 × 10−19
E) 2.5
A) 4.2
B) 4.0 × 10−19
C) 4.0 × 10−10
D) 2.5 × 10−19
E) 2.5

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
19
How much energy is in an 89.7 MHz photon of FM-radiation?
A) 2.2 × 10−33 J
B) 9.5 × 10−27 J
C) 7.4 × 10−42 J
D) 5.9 × 10−26 J
E) 3.7 × 10−25 J
A) 2.2 × 10−33 J
B) 9.5 × 10−27 J
C) 7.4 × 10−42 J
D) 5.9 × 10−26 J
E) 3.7 × 10−25 J

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
20
The light intensity incident on a metallic surface produces photoelectrons with a maximum kinetic energy of 2 eV. The light intensity is doubled. Determine the maximum kinetic energy of the photoelectrons (in eV).
A) 4
B) 2
C)
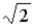
D) 3
E) 16
A) 4
B) 2
C)
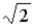
D) 3
E) 16

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
21
A quantum particle
A) can be localized in space.
B) can be represented by an infinitely long wave having a single frequency.
C) can be represented by a wave packet.
D) travels at the phase speed of the infinitely long wave having the highest frequency.
E) has the highest probability of being present in those regions of space where its component waves interfere destructively.
A) can be localized in space.
B) can be represented by an infinitely long wave having a single frequency.
C) can be represented by a wave packet.
D) travels at the phase speed of the infinitely long wave having the highest frequency.
E) has the highest probability of being present in those regions of space where its component waves interfere destructively.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
22
Film behind a double slit is exposed to light in the following way: First one slit is opened and light is allowed to go through that slit for time Δt. Then it is closed and the other slit is opened and light is allowed to go through that slit for the same time Δt. When the film is developed the pattern will be
A) one single slit pattern.
B) two superimposed single slit patterns, their centers displaced from each other by the distance between the two slits.
C) one double slit pattern.
D) two double slit patterns, their centers displaced from each other by the distance between the two slits.
E) random darkening of the film. (no pattern at all)
A) one single slit pattern.
B) two superimposed single slit patterns, their centers displaced from each other by the distance between the two slits.
C) one double slit pattern.
D) two double slit patterns, their centers displaced from each other by the distance between the two slits.
E) random darkening of the film. (no pattern at all)

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
23
The number of photons per second passing a plane perpendicular to a collimated monochromatic (one frequency) beam of light transporting power P is directly proportional to
A) the wavelength of the light.
B) the frequency of the light.
C) the power of the beam.
D) all of the above.
E) only (a) and (c) above.
A) the wavelength of the light.
B) the frequency of the light.
C) the power of the beam.
D) all of the above.
E) only (a) and (c) above.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
24
A baseball (1 kg) has an energy of 100 joules. If its uncertainty in position is 1 m, what is the percentage uncertainty (Δp/p × 100) in the momentum of the baseball?
A) <<1%
B) 1%
C) 2%
D) 5%
E) 10%
A) <<1%
B) 1%
C) 2%
D) 5%
E) 10%

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
25
The wavelength of a 45-kg teenager moving at 5 m/s on a skateboard is about
A) 5 × 10−37 m.
B) 3 × 10−36 m.
C) 7 × 10−34 m.
D) 0.1 m.
E) 5 m.
A) 5 × 10−37 m.
B) 3 × 10−36 m.
C) 7 × 10−34 m.
D) 0.1 m.
E) 5 m.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
26
Because the factor h on the right side of the Heisenberg uncertainty principle has units of Joule-seconds, it suggests that the energy of a system also has uncertainty. The uncertainty in energy depends on the length of the time interval during which a system exists. ΔEΔt ≥ h/4π. Suppose an unstable mass is produced during a high-energy collision such that the uncertainty in its mass is me/100. (me = 9.11 × 10−31 kg.) How long will this particle exist?
A) 6.4 × 10−20 s
B) 2.3 × 10−23 s
C) 1.0 × 1015 s
D) 1.2 × 1013 s
E) 8.1 × 10−19 s
A) 6.4 × 10−20 s
B) 2.3 × 10−23 s
C) 1.0 × 1015 s
D) 1.2 × 1013 s
E) 8.1 × 10−19 s

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
27
Assume we can determine the position of a particle within an uncertainty of 0.5 nm. What will be the resulting uncertainty in the particle's momentum (in kg ⋅ m/s)?
A) 1.9 × 10−25
B) 4.2 × 10−25
C) 1.1 × 10−25
D) 1.3 × 10−24
E) 6.6 × 10−25
A) 1.9 × 10−25
B) 4.2 × 10−25
C) 1.1 × 10−25
D) 1.3 × 10−24
E) 6.6 × 10−25

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
28
A pendulum located where 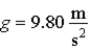 has a 0.500 kg bob and a 4.00 Hz oscillation frequency. If it is released from a point 0.150 m above the lowest point it reaches, the number of quanta in this oscillation is
has a 0.500 kg bob and a 4.00 Hz oscillation frequency. If it is released from a point 0.150 m above the lowest point it reaches, the number of quanta in this oscillation is
A) 2.77 × 1032.
B) 1.11 × 1033.
C) 4.87 × 10−34.
D) 0.184.
E) 4.00.
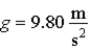 has a 0.500 kg bob and a 4.00 Hz oscillation frequency. If it is released from a point 0.150 m above the lowest point it reaches, the number of quanta in this oscillation is
has a 0.500 kg bob and a 4.00 Hz oscillation frequency. If it is released from a point 0.150 m above the lowest point it reaches, the number of quanta in this oscillation isA) 2.77 × 1032.
B) 1.11 × 1033.
C) 4.87 × 10−34.
D) 0.184.
E) 4.00.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
29
Photoelectrons are ejected when monochromatic light shines on a freshly-prepared (oxide-free) sodium surface. In order to obtain the maximum increase in the number of electrons ejected per second, the experimenter needs to
A) increase the frequency of the light.
B) increase the intensity of the light.
C) increase the area illuminated by the light.
D) do all of the above.
E) do only (b) and (c) above.
A) increase the frequency of the light.
B) increase the intensity of the light.
C) increase the area illuminated by the light.
D) do all of the above.
E) do only (b) and (c) above.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
30
In electron diffraction, an electron moving at speed v << c acts like
A) a particle with momentum mv.
B) a particle with position coordinate v/t.
C) a wave with wavelength v/t.
D) a wave with wavelength h/mv.
E) both a particle with position coordinate v/t and a wave with wavelength v/t.
A) a particle with momentum mv.
B) a particle with position coordinate v/t.
C) a wave with wavelength v/t.
D) a wave with wavelength h/mv.
E) both a particle with position coordinate v/t and a wave with wavelength v/t.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
31
The experimental observation(s) below that require(s) a quantum explanation for the photoelectric effect
A) is that more photoelectrons are emitted when the light frequency increases.
B) is that the maximum kinetic energy of the photoelectrons is related linearly to the frequency of the light.
C) is that every metal surface has a work function, a minimum amount of energy needed to free electrons.
D) is that the stopping potential measures the kinetic energy of the photoelectrons.
E) are all of the above.
A) is that more photoelectrons are emitted when the light frequency increases.
B) is that the maximum kinetic energy of the photoelectrons is related linearly to the frequency of the light.
C) is that every metal surface has a work function, a minimum amount of energy needed to free electrons.
D) is that the stopping potential measures the kinetic energy of the photoelectrons.
E) are all of the above.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
32
A wave packet can represent a quantum particle because
A) it has the localized nature of a particle.
B) the group velocity is identical to the speed of the particle.
C) the waves that compose the packet can show interference and diffraction.
D) of all of the above.
E) of only (a) and (b) above.
A) it has the localized nature of a particle.
B) the group velocity is identical to the speed of the particle.
C) the waves that compose the packet can show interference and diffraction.
D) of all of the above.
E) of only (a) and (b) above.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
33
When a photon collides with a free electron at rest and the direction of motion of the photon changes,
A) the magnitude of the momentum of the photon does not change.
B) the momentum of the electron does not change.
C) the kinetic energy of the electron does not change.
D) the total energy of the photon does not change.
E) both the magnitude of the momentum and the total energy of the photon decrease.
A) the magnitude of the momentum of the photon does not change.
B) the momentum of the electron does not change.
C) the kinetic energy of the electron does not change.
D) the total energy of the photon does not change.
E) both the magnitude of the momentum and the total energy of the photon decrease.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
34
In an experiment different wavelengths of light, all able to eject photoelectrons, shine on a freshly prepared (oxide-free) zinc surface. Which statement is true?
A) The number of photoelectrons emitted per second is independent of the intensity of the light for all the different wavelengths.
B) The number of photoelectrons emitted per second is directly proportional to the frequency for all the different wavelengths.
C) The maximum kinetic energy of the photoelectrons emitted is directly proportional to the frequency for each wavelength present.
D) The maximum kinetic energy of the photoelectrons has a linear relationship with the frequency for each wavelength present.
E) The maximum kinetic energy of the photoelectrons is proportional to the intensity of the light and independent of the frequency.
A) The number of photoelectrons emitted per second is independent of the intensity of the light for all the different wavelengths.
B) The number of photoelectrons emitted per second is directly proportional to the frequency for all the different wavelengths.
C) The maximum kinetic energy of the photoelectrons emitted is directly proportional to the frequency for each wavelength present.
D) The maximum kinetic energy of the photoelectrons has a linear relationship with the frequency for each wavelength present.
E) The maximum kinetic energy of the photoelectrons is proportional to the intensity of the light and independent of the frequency.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
35
Find the uncertainty in the momentum (in kg ⋅ m/s) of an electron if the uncertainty in its position is equal to 3.4 × 10−10 m, the circumference of the first Bohr orbit.
A) 6.2 × 10−25
B) 1.6 × 10−25
C) 15.5 × 10−25
D) 19.4 × 10−25
E) 2.0 × 10−24
A) 6.2 × 10−25
B) 1.6 × 10−25
C) 15.5 × 10−25
D) 19.4 × 10−25
E) 2.0 × 10−24

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
36
Assume the Heisenberg uncertainty principle can take the form ΔEΔt ≥ h/4B. How accurate can the position of an electron be made if its speed is 5 × 106 m/s and if the uncertainty in its energy is 10 eV?
A) 7.7 × 10−18 m
B) 1.3 × 10−23 m
C) 6.2 × 10−15 m
D) 1.6 × 10−10 m
E) 1.2 × 10−9 m
A) 7.7 × 10−18 m
B) 1.3 × 10−23 m
C) 6.2 × 10−15 m
D) 1.6 × 10−10 m
E) 1.2 × 10−9 m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
37
An electron has been accelerated by a potential difference of 100 V. If its position is known to have an uncertainty of 1 nm, what is the percent uncertainty (Δp/p × 100) of the electron?
A) 1%
B) 2%
C) 10%
D) >>10%
E) 5%
A) 1%
B) 2%
C) 10%
D) >>10%
E) 5%

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
38
Microscopes are inherently limited by the wavelength of the light used. How much smaller (in order of magnitude) can we "see" using an electron microscope whose electrons have been accelerated through a potential difference of 50000 V than using red light (500 nm)?
A) 3
B) 4
C) 5
D) 6
E) 14
A) 3
B) 4
C) 5
D) 6
E) 14

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
39
Because of Heisenberg's Uncertainty Principle, the velocity of a particle is known least precisely when the length of the wave packet representing the particle is
A) < 10−15 m.
B) < 10−10 m.
C) of the order of 1.00 m.
D) >10 m.
E) the length of a plane wave (infinite)
A) < 10−15 m.
B) < 10−10 m.
C) of the order of 1.00 m.
D) >10 m.
E) the length of a plane wave (infinite)

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
40
If the position of an electron (m = 9.11 × 10−31 kg) could be measured to within 10−30 m, the uncertainty in the magnitude of its speed could be as much as
A) 6 × 10−34 m/s.
B) 6 × 1025 m/s.
C) 6 × 1030 m/s.
D) 1031 m/s.
E) 1061 m/s.
A) 6 × 10−34 m/s.
B) 6 × 1025 m/s.
C) 6 × 1030 m/s.
D) 1031 m/s.
E) 1061 m/s.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
41
If a rubidium surface is irradiated with blue light of wavelength 450.0 nm, what is the kinetic energy of the electrons emitted? (The work function for rubidium is φ = 2.09 eV.)

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
42
An electron is sitting on a pinpoint having a diameter of 2.5 μm. What is the minimum uncertainty in the speed of the electron?

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
43
The "seeing ability" or resolution of radiation is determined by its wavelength. If an atom is approximately 10−10 m in diameter, how fast must an electron travel to have a wavelength smaller than the size of an atom?

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
44
What is the shortest x-ray wavelength that can be produced in a 12-keV x-ray machine?

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
45
On a bright and sunny day, the intensity of solar radiation on the Earth's surface is 1 000 W/m2. If the average wavelength of the sunlight is 500 nm, how many photons are incident on a square meter of the Earth's surface per second?

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
46
What is the energy in eV of a photon of yellow light? λ = 500 nm.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
47
An atomic level has a lifetime, τ, of 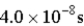 . What is the linewidth,
. What is the linewidth, 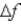 , of this level?
, of this level?
A)
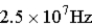
B)
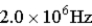
C)
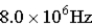
D)
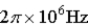
E)
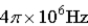
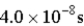 . What is the linewidth,
. What is the linewidth, 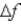 , of this level?
, of this level?A)
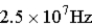
B)
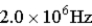
C)
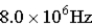
D)
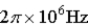
E)
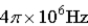

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
48
Suppose we use optical radiation (λ = 500 nm) to determine the position of the electron to within the wavelength of the light. What will be the resulting uncertainty in the electron's velocity?

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck


