Exam 40: Introduction to Quantum Physics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
What is the shortest x-ray wavelength that can be produced in a 12-keV x-ray machine?
Free
(Essay)
4.8/5  (20)
(20)
Correct Answer:
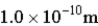
Assume we can determine the position of a particle within an uncertainty of 0.5 nm. What will be the resulting uncertainty in the particle's momentum (in kg ⋅ m/s)?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
C
What is the maximum kinetic energy (in eV) of a photoelectron when a surface, whose work function is 5.0 eV, is illuminated by photons whose wavelength is 400 nm?
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
D
In electron diffraction, an electron moving at speed v << c acts like
(Multiple Choice)
4.9/5  (32)
(32)
If the position of an electron (m = 9.11 × 10−31 kg) could be measured to within 10−30 m, the uncertainty in the magnitude of its speed could be as much as
(Multiple Choice)
4.7/5  (30)
(30)
The light intensity incident on a metallic surface with a work function of 3 eV produces photoelectrons with a maximum kinetic energy of 2 eV. The frequency of the light is doubled. Determine the maximum kinetic energy (in eV).
(Multiple Choice)
4.7/5  (28)
(28)
Find the uncertainty in the momentum (in kg ⋅ m/s) of an electron if the uncertainty in its position is equal to 3.4 × 10−10 m, the circumference of the first Bohr orbit.
(Multiple Choice)
4.9/5  (43)
(43)
An electron is sitting on a pinpoint having a diameter of 2.5 μm. What is the minimum uncertainty in the speed of the electron?
(Short Answer)
4.8/5  (28)
(28)
When a photon collides with a free electron at rest and the direction of motion of the photon changes,
(Multiple Choice)
4.9/5  (35)
(35)
A helium-neon laser emits red light having a wavelength of 6.4 × 10−7 m and a power of 0.5 mW. How many photons are emitted each second?
(Multiple Choice)
4.8/5  (22)
(22)
If a rubidium surface is irradiated with blue light of wavelength 450.0 nm, what is the kinetic energy of the electrons emitted? (The work function for rubidium is φ = 2.09 eV.)
(Short Answer)
4.9/5  (26)
(26)
A photon collides with an electron. After the collision the wavelength of the scattered wave at a scattering angle greater than 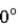 is
is
(Multiple Choice)
4.9/5  (33)
(33)
What is the maximum kinetic energy (in eV) of a photoelectron emitted from a surface whose work function is 5.0 eV when illuminated by a light whose wavelength is 200 nm?
(Multiple Choice)
4.8/5  (20)
(20)
A solid state pulsed laser has an energy of 400 mJ per pulse. If its wavelength is 1.06 × 10−6 m, how many photons are in each pulse?
(Multiple Choice)
4.8/5  (38)
(38)
A neutron has a mass of 1.67 × 10−27 kg. Its de Broglie wavelength is 1.4 × 10−10 m. What temperature would it correspond to if we had a monatomic gas having the same average kinetic energy (in °C)?
(Multiple Choice)
4.7/5  (34)
(34)
Microscopes are inherently limited by the wavelength of the light used. How much smaller (in order of magnitude) can we "see" using an electron microscope whose electrons have been accelerated through a potential difference of 50000 V than using red light (500 nm)?
(Multiple Choice)
4.7/5  (34)
(34)
The light intensity incident on a metallic surface produces photoelectrons with a maximum kinetic energy of 2 eV. The light intensity is doubled. Determine the maximum kinetic energy of the photoelectrons (in eV).
(Multiple Choice)
4.8/5  (33)
(33)
The "seeing ability" or resolution of radiation is determined by its wavelength. If an atom is approximately 10−10 m in diameter, how fast must an electron travel to have a wavelength smaller than the size of an atom?
(Short Answer)
4.7/5  (26)
(26)
A stopping potential of 3.2 V is needed for radiation whose wavelength is 200 nm. What is the work function (in eV) of the material?
(Multiple Choice)
5.0/5  (33)
(33)
Showing 1 - 20 of 48
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)