Deck 7: Reaction Rates and Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/104
Play
Full screen (f)
Deck 7: Reaction Rates and Chemical Equilibrium
1
For which of the following reactions is it important that the species collide with the correct orientation?
A) HCl(g) + H2O(l) → H2O+(aq) + Cl-(aq)
B) Ag+(aq) + Cl-(aq) → AgCl(s)
C) both of these
D) neither of these
A) HCl(g) + H2O(l) → H2O+(aq) + Cl-(aq)
B) Ag+(aq) + Cl-(aq) → AgCl(s)
C) both of these
D) neither of these
C
2
Which of the following statements is true of the reaction A + B → 2C?
A) B is consumed at the same rate that A is consumed.
B) C is produced at the same rate A is consumed.
C) C is produced at the same rate B is consumed.
D) B is produced at the same rate A is consumed.
A) B is consumed at the same rate that A is consumed.
B) C is produced at the same rate A is consumed.
C) C is produced at the same rate B is consumed.
D) B is produced at the same rate A is consumed.
A
3
In an energy diagram for a chemical reaction, what species represents the highest energy?
A) the catalyst
B) the products
C) the reactants
D) the transition state
A) the catalyst
B) the products
C) the reactants
D) the transition state
D
4
Which of the following is the study of the rates of chemical reactions?
A) kinetics
B) stoichiometry
C) thermodynamics
D) none of these
A) kinetics
B) stoichiometry
C) thermodynamics
D) none of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is true of the rates of most chemical reactions?
A) The initial rate cannot be measured.
B) The initial rate is faster than the rate later in time.
C) The initial rate is the same as the rate later in time.
D) The initial rate is slower than the rate later in time.
A) The initial rate cannot be measured.
B) The initial rate is faster than the rate later in time.
C) The initial rate is the same as the rate later in time.
D) The initial rate is slower than the rate later in time.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
6
For the reaction 2C → A + B, 0.02 mol of A is formed during the first 15 seconds of the reaction. Assuming that the rate of reaction remains constant for 2 min, which of the following statements is true?
A) After 2 min, 0.16 mol of B was produced.
B) After 2 min, 0.32 mol of C was consumed.
C) After 2 min, 0.16 mol of B was produced and .32 mol of C was consumed.
D) None of these.
A) After 2 min, 0.16 mol of B was produced.
B) After 2 min, 0.32 mol of C was consumed.
C) After 2 min, 0.16 mol of B was produced and .32 mol of C was consumed.
D) None of these.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
7
For the reaction 2HgO(s) → 2Hg(l) + O2(g), we measure the evolution of gas to determine the rate of reaction. At the beginning of the reaction (at 0 min), 0.070 L of O2 is present. After 15 min, the volume of O2 is 0.40 L. What is the rate of reaction?
A) 0.022 L/min
B) 0.033 L/min
C) 0.23 L/min
D) 0.33 L/min
A) 0.022 L/min
B) 0.033 L/min
C) 0.23 L/min
D) 0.33 L/min

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is true of activation energy?
A) Reactions with low activation energies are rapid.
B) The activation energy for an exothermic reaction is negative.
C) The activation energy is the energy difference between the reactants and the products.
D) Reactions with low activation energies have lower number of effective collisions.
A) Reactions with low activation energies are rapid.
B) The activation energy for an exothermic reaction is negative.
C) The activation energy is the energy difference between the reactants and the products.
D) Reactions with low activation energies have lower number of effective collisions.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following units can used to describe the rate of a chemical reaction?
A) (mol·L)/min
B) (mol/L)/min
C) 1/(mol·L·min)
D) (mol·L)·min
A) (mol·L)/min
B) (mol/L)/min
C) 1/(mol·L·min)
D) (mol·L)·min

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is not true of activation energy?
A) Decreasing the activation energy decreases the reaction rate.
B) Decreasing the activation energy increases the reaction rate.
C) The activation energy is the minimum energy required for a reaction to occur.
D) The activation energy of an endothermic reaction is positive.
A) Decreasing the activation energy decreases the reaction rate.
B) Decreasing the activation energy increases the reaction rate.
C) The activation energy is the minimum energy required for a reaction to occur.
D) The activation energy of an endothermic reaction is positive.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements is true of the reaction A + B → 2C?
A) B is consumed at the same rate that A is consumed.
B) C is produced at twice the rate A is consumed.
C) B is consumed at the same rate that A is consumed and C is produced at twice the rate A is consumed.
D) None of these are true.
A) B is consumed at the same rate that A is consumed.
B) C is produced at twice the rate A is consumed.
C) B is consumed at the same rate that A is consumed and C is produced at twice the rate A is consumed.
D) None of these are true.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is true of effective collisions?
A) The number of effective collisions decreases as the temperature increases.
B) The number of effective collisions determines the rate of a reaction.
C) The number of effective collisions is independent of the orientation of the molecules.
D) The number of effective collisions determines the phases of the reactants.
A) The number of effective collisions decreases as the temperature increases.
B) The number of effective collisions determines the rate of a reaction.
C) The number of effective collisions is independent of the orientation of the molecules.
D) The number of effective collisions determines the phases of the reactants.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
13
For the reaction A + B → 2C, which of the following best describes how we can measure the reaction rate?
A) The rate can be measured by measuring how fast A is produced.
B) The rate can be measured by measuring how fast C is produced.
C) The rate can be measured by measuring how fast B is produced.
D) The rate can be measured by measuring how fast C is consumed.
A) The rate can be measured by measuring how fast A is produced.
B) The rate can be measured by measuring how fast C is produced.
C) The rate can be measured by measuring how fast B is produced.
D) The rate can be measured by measuring how fast C is consumed.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is not true of effective collisions?
A) The number of effective collisions does not affect the reaction rate.
B) The number of effective collisions increases as the temperature increases.
C) The number of effective collisions is dependent on the orientation of the molecules.
D) The number of effective collisions is dependent on the overall frequency of collisions.
A) The number of effective collisions does not affect the reaction rate.
B) The number of effective collisions increases as the temperature increases.
C) The number of effective collisions is dependent on the orientation of the molecules.
D) The number of effective collisions is dependent on the overall frequency of collisions.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
15
Many molecular collisions do not result in chemical reactions. Why?
A) The colliding molecules are not the correct chemicals.
B) The colliding molecules do not have sufficient energy.
C) The colliding molecules do not have the correct orientations.
D) All of these are reasons many molecular collisions do not result in chemical reactions.
A) The colliding molecules are not the correct chemicals.
B) The colliding molecules do not have sufficient energy.
C) The colliding molecules do not have the correct orientations.
D) All of these are reasons many molecular collisions do not result in chemical reactions.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
16
For the reaction 2C → A + B, 0.02 mol of A is formed during the first 15 seconds of the reaction. Assuming that the rate of reaction remains constant for 2 min, which of the following statements is true?
A) After 2 min, 0.16 mol of B was produced.
B) After 2 min, 0.16 mol of C was consumed.
C) After 2 min, 0.16 mol of B was produced and .16 mol of C was consumed.
D) None of these.
A) After 2 min, 0.16 mol of B was produced.
B) After 2 min, 0.16 mol of C was consumed.
C) After 2 min, 0.16 mol of B was produced and .16 mol of C was consumed.
D) None of these.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
17
For which of the following reactions is it important that the species collide with the correct orientation?
A) 2NO(g) + O2(g) → 2NO2(g)
B) HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq)
C) both of these
D) neither of these
A) 2NO(g) + O2(g) → 2NO2(g)
B) HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq)
C) both of these
D) neither of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following best describes the rates of chemical reactions?
A) Most chemical reactions occur very slowly.
B) Most chemical reactions occur at moderate rates.
C) Most chemical reactions occur very rapidly.
D) Chemical reactions have a wide range of rates, from extremely fast to extremely slow.
A) Most chemical reactions occur very slowly.
B) Most chemical reactions occur at moderate rates.
C) Most chemical reactions occur very rapidly.
D) Chemical reactions have a wide range of rates, from extremely fast to extremely slow.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
19
For which of the following reactions is it important that the species collide with the correct orientation?
A) 2H2(g) + O2(g) → 2H2O(l)
B) HCl(g) + H2O(l) → H2O+(aq) + Cl-(aq)
C) both of these
D) neither of these
A) 2H2(g) + O2(g) → 2H2O(l)
B) HCl(g) + H2O(l) → H2O+(aq) + Cl-(aq)
C) both of these
D) neither of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
20
Why will increasing the temperature of a reaction speed up the reaction?
A) because the activation energy for the reaction is lowered
B) because the heat of reaction is increased
C) because there are more effective collisions between molecules
D) none of these
A) because the activation energy for the reaction is lowered
B) because the heat of reaction is increased
C) because there are more effective collisions between molecules
D) none of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
21
If a chemist wishes to react a solid with a liquid, which of the following will be the least effective in speeding up the reaction?
A) adding more liquid reactant
B) grinding the solid to a form a powder
C) dissolving the solid and the liquid in a solvent
D) using the frozen form of the liquid
A) adding more liquid reactant
B) grinding the solid to a form a powder
C) dissolving the solid and the liquid in a solvent
D) using the frozen form of the liquid

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following accounts for the fact that reactions between ions in solution are usually very fast?
A) Ionic bonds are weak.
B) No covalent bonds need to be broken for a reaction to occur.
C) Reactions between ions are always endothermic.
D) Reactions between ions are always exothermic.
A) Ionic bonds are weak.
B) No covalent bonds need to be broken for a reaction to occur.
C) Reactions between ions are always endothermic.
D) Reactions between ions are always exothermic.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
23
Given that the reaction C(s) + O2(g) → CO2(g) is exothermic, which of the following is true of the reaction CO2(g) → C(s) + O2(g)?
A) Its activation energy is lower than that of C(s) + O2(g) → CO2(g).
B) Its activation energy is the same as that of C(s) + O2(g) → CO2(g).
C) Its activation energy is higher than that of C(s) + O2(g) → CO2(g).
D) There is no relationship between its activation energy and that of C(s) + O2(g) → CO2(g).
A) Its activation energy is lower than that of C(s) + O2(g) → CO2(g).
B) Its activation energy is the same as that of C(s) + O2(g) → CO2(g).
C) Its activation energy is higher than that of C(s) + O2(g) → CO2(g).
D) There is no relationship between its activation energy and that of C(s) + O2(g) → CO2(g).

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
24
In an energy diagram for an endothermic chemical reaction, which of the following is true?
A) The energy of the products is lower than that of the reactants.
B) The energy of the transition state is higher than that of the reactants.
C) The energy of the products is lower that that of the reactants and the energy of the transition state is higher than that of the reactants.
D) None of these.
A) The energy of the products is lower than that of the reactants.
B) The energy of the transition state is higher than that of the reactants.
C) The energy of the products is lower that that of the reactants and the energy of the transition state is higher than that of the reactants.
D) None of these.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
25
The rate of reaction for the decomposition of hydrogen peroxide to yield water and oxygen is represented by the following equation: Rate = k[H2O2]. Which of the following is indicated by this equation?
A) The rate of the reaction is unaffected by the concentration of O2.
B) The rate of the reaction will increase with the increasing concentration of H2O2.
C) The rate of the reaction will decrease with the increasing concentration of O2.
D) None of these are indicated by the equation.
A) The rate of the reaction is unaffected by the concentration of O2.
B) The rate of the reaction will increase with the increasing concentration of H2O2.
C) The rate of the reaction will decrease with the increasing concentration of O2.
D) None of these are indicated by the equation.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
26
If a reaction occurs very rapidly even at a relatively low temperature, which of the following is true?
A) The reaction is endothermic.
B) The reaction is exothermic.
C) The reaction has a low activation energy.
D) The reaction must be a single-step reaction.
A) The reaction is endothermic.
B) The reaction is exothermic.
C) The reaction has a low activation energy.
D) The reaction must be a single-step reaction.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
27
In an energy diagram for an exothermic chemical reaction, which of the following is true?
A) The energy of the products is lower than that of the reactants.
B) The energy of the transition state is higher than that of the reactants.
C) The energy of the products is lower than that of the reactants and the energy of the transition state is higher than that of the reactants.
D) None of these.
A) The energy of the products is lower than that of the reactants.
B) The energy of the transition state is higher than that of the reactants.
C) The energy of the products is lower than that of the reactants and the energy of the transition state is higher than that of the reactants.
D) None of these.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following does not result from increasing the temperature of a reaction?
A) The fraction of effective collisions increases.
B) The concentrations of the reactants increase.
C) There are more collisions.
D) None, all of these are a result of increasing the temperature.
A) The fraction of effective collisions increases.
B) The concentrations of the reactants increase.
C) There are more collisions.
D) None, all of these are a result of increasing the temperature.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
29
Given that the reaction 2Na2O (s) → 4Na(s) + 2O2(g) is endothermic, which of the following is true of the reaction 4Na(s) + 2O2(g) → 2Na2O(s)?
A) Its activation energy is lower than that of 2Na2O(s) → 4Na(s) + 2O2(g).
B) Its activation energy is the same as that of 2Na2O(s) → 4Na(s) + 2O2(g).
C) Its activation energy is higher than that of 2Na2O(s) → 4Na(s) + 2O2(g).
D) There is no relationship between its activation energy and that of 2Na2O(s) → 4Na(s) + 2O2(g).
A) Its activation energy is lower than that of 2Na2O(s) → 4Na(s) + 2O2(g).
B) Its activation energy is the same as that of 2Na2O(s) → 4Na(s) + 2O2(g).
C) Its activation energy is higher than that of 2Na2O(s) → 4Na(s) + 2O2(g).
D) There is no relationship between its activation energy and that of 2Na2O(s) → 4Na(s) + 2O2(g).

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
30
Potassium hydroxide and phosphorus pentachloride react to form potassium phosphate and potassium chloride. Which of the following will result in the fastest reaction between potassium hydroxide and phosphorus pentachloride?
A) Crystals of the two reactants are placed in a mortar and are ground.
B) Large crystals of the two reactants are placed in contact with one another.
C) Powdered samples of the two reactants are mixed together.
D) Solutions of the two reactants are mixed together.
A) Crystals of the two reactants are placed in a mortar and are ground.
B) Large crystals of the two reactants are placed in contact with one another.
C) Powdered samples of the two reactants are mixed together.
D) Solutions of the two reactants are mixed together.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is true of most multistep reactions?
A) Most of them involve the participation of only two reactant molecules.
B) They are all endothermic reactions.
C) They are all exothermic reactions.
D) The rate of reaction is usually determined by the slowest step.
A) Most of them involve the participation of only two reactant molecules.
B) They are all endothermic reactions.
C) They are all exothermic reactions.
D) The rate of reaction is usually determined by the slowest step.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
32
How does the rate of reaction change as the concentration of the product increases over the course of a reaction?
A) The rate of reaction always decreases.
B) The rate of reaction usually decreases.
C) The rate of reaction always increases.
D) The rate of reaction usually increases.
A) The rate of reaction always decreases.
B) The rate of reaction usually decreases.
C) The rate of reaction always increases.
D) The rate of reaction usually increases.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
33
Consider a reaction such as A2(g) + B2(g) → 2AB(g). What can we say about the mechanism of the reaction?
A) If the reaction is fast, it occurs in a single step.
B) If the reaction is fast, it occurs in multiple steps.
C) If the reaction is slow, it occurs in multiple steps.
D) We must examine each case individually.
A) If the reaction is fast, it occurs in a single step.
B) If the reaction is fast, it occurs in multiple steps.
C) If the reaction is slow, it occurs in multiple steps.
D) We must examine each case individually.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
34
In a particular chemical reaction, three bonds are broken and four bonds are formed. Based on this information alone, which of the following is true?
A) The reaction has a high activation energy.
B) The reaction is endothermic.
C) The reaction is exothermic.
D) We cannot determine which of the statements is true.
A) The reaction has a high activation energy.
B) The reaction is endothermic.
C) The reaction is exothermic.
D) We cannot determine which of the statements is true.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
35
How does the rate of reaction change when the reactant is used up over the course of a reaction?
A) The rate of reaction always decreases.
B) The rate of reaction usually decreases.
C) The rate of reaction always increases.
D) The rate of reaction usually increases.
A) The rate of reaction always decreases.
B) The rate of reaction usually decreases.
C) The rate of reaction always increases.
D) The rate of reaction usually increases.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
36
The reaction H2O2(l) + 3I-(aq) + 2 H+(aq) → I3-(aq) + 2H2O(aq) occurs very rapidly. Based on this observation alone, what can we say about this reaction?
A) The reaction probably occurs as a single fast step.
B) The reaction probably occurs as a series of steps, each of which is fast.
C) The reaction is endothermic.
D) The reaction is exothermic.
A) The reaction probably occurs as a single fast step.
B) The reaction probably occurs as a series of steps, each of which is fast.
C) The reaction is endothermic.
D) The reaction is exothermic.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
37
In a particular chemical reaction, four bonds are broken and three bonds are formed. Based only on this information, which of the following is true?
A) The reaction has a high activation energy.
B) The reaction is endothermic.
C) The reaction is exothermic.
D) We cannot determine which of the statements is true.
A) The reaction has a high activation energy.
B) The reaction is endothermic.
C) The reaction is exothermic.
D) We cannot determine which of the statements is true.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
38
Solid sodium chloride and solid silver nitrate react to form sodium nitrate and silver chloride. The reaction is extremely slow. However, if the sodium chloride and silver nitrate are first dissolved in water and the two solutions are mixed, the reaction occurs very rapidly. Which of the following explains the difference in reaction rates?
A) Ions in solution have high mobility, and ions of opposite charges are attracted to each other.
B) The concentration of ions in solution is greater than the concentration of ions in the solid.
C) Sodium chloride solution does not react with solid silver nitrate.
D) Silver nitrate solution does not react with solid sodium chloride.
A) Ions in solution have high mobility, and ions of opposite charges are attracted to each other.
B) The concentration of ions in solution is greater than the concentration of ions in the solid.
C) Sodium chloride solution does not react with solid silver nitrate.
D) Silver nitrate solution does not react with solid sodium chloride.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
39
In a particular chemical reaction, two bonds are broken and no bonds are formed. Based on this information alone, which of the following is true?
A) The reaction has a high activation energy.
B) The reaction is endothermic.
C) The reaction is exothermic.
D) We cannot determine which of the statements is true.
A) The reaction has a high activation energy.
B) The reaction is endothermic.
C) The reaction is exothermic.
D) We cannot determine which of the statements is true.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
40
If a chemist wishes to react a solid with a liquid, which of the following will be the most effective in speeding up the reaction?
A) adding more liquid reactant
B) grinding the solid to a form a powder
C) placing a large crystal of the solid in a beaker containing the liquid reactant
D) all of these
A) adding more liquid reactant
B) grinding the solid to a form a powder
C) placing a large crystal of the solid in a beaker containing the liquid reactant
D) all of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
41
What is the effect of increasing the temperature at which a reaction is carried out?
A) In all cases, the rate of reaction decreases.
B) In virtually all cases, the rate of reaction decreases.
C) In all cases, the rate of reaction increases.
D) In virtually all cases, the rate of reaction increases.
A) In all cases, the rate of reaction decreases.
B) In virtually all cases, the rate of reaction decreases.
C) In all cases, the rate of reaction increases.
D) In virtually all cases, the rate of reaction increases.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
42
Consider a sample of water in a closed, rigid container. Which of the following will indicate that the given physical process has reached equilibrium? 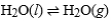
A) The mass of the container remains constant.
B) The vapor pressure in the container remains constant.
C) The volume of water in the container increases from its original level.
D) All of these indicate that the reaction has reached equilibrium.
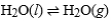
A) The mass of the container remains constant.
B) The vapor pressure in the container remains constant.
C) The volume of water in the container increases from its original level.
D) All of these indicate that the reaction has reached equilibrium.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is an example of an equilibrium situation?
A) an unsaturated solution
B) a saturated solution
C) a supersaturated solution
D) all of these
A) an unsaturated solution
B) a saturated solution
C) a supersaturated solution
D) all of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
44
If a particular reactant is involved in the slowest step of a multistep reaction, then what will be the effect of increasing its concentration?
A) The reaction rate will decrease.
B) The reaction rate will increase.
C) The reaction rate will be unaffected.
A) The reaction rate will decrease.
B) The reaction rate will increase.
C) The reaction rate will be unaffected.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following statements is true of a reversible reaction?
A) All reversible reactions are exothermic.
B) All reversible reactions are endothermic.
C) When we mix the reactants together, one or more of the reactants will be completely used up.
D) When we mix the reactants together, none of the reactants will be completely used up.
A) All reversible reactions are exothermic.
B) All reversible reactions are endothermic.
C) When we mix the reactants together, one or more of the reactants will be completely used up.
D) When we mix the reactants together, none of the reactants will be completely used up.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
46
What is the effect of decreasing the temperature at which a reaction is carried out?
A) In all cases, the rate of reaction decreases.
B) In virtually all cases, the rate of reaction decreases.
C) In all cases, the rate of reaction increases.
D) In virtually all cases, the rate of reaction increases.
A) In all cases, the rate of reaction decreases.
B) In virtually all cases, the rate of reaction decreases.
C) In all cases, the rate of reaction increases.
D) In virtually all cases, the rate of reaction increases.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
47
A catalyst speeds up a chemical reaction as a result of which of the following?
A) It provides an alternate pathway with a lower activation energy.
B) It increases the number of collisions among reacting molecules.
C) It makes the reaction more endothermic.
D) It makes the reaction more exothermic.
A) It provides an alternate pathway with a lower activation energy.
B) It increases the number of collisions among reacting molecules.
C) It makes the reaction more endothermic.
D) It makes the reaction more exothermic.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
48
In a certain chemical reaction, 1.00 g of product is produced in 15 min when the reaction is carried out at 20°C. Assuming that there is sufficient starting material available, approximately how much product will be produced in the same length of time if the reaction is carried out in a boiling water bath at 100°C?
A) 2.00 g
B) 16.0 g
C) 64.0 g
D) 256 g
A) 2.00 g
B) 16.0 g
C) 64.0 g
D) 256 g

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
49
A catalyst speeds up a chemical reaction as a result of which of the following?
A) It increases the number of collisions among reacting molecules.
B) It makes the reaction more endothermic.
C) It makes the reaction more exothermic.
D) It provides an alternative reaction pathway.
A) It increases the number of collisions among reacting molecules.
B) It makes the reaction more endothermic.
C) It makes the reaction more exothermic.
D) It provides an alternative reaction pathway.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is true of fevers?
A) An increase in body temperature never has beneficial effects.
B) A 1°C increase in temperature is a protective mechanism.
C) A 5°C increase in temperature is never fatal.
D) None of these are true.
A) An increase in body temperature never has beneficial effects.
B) A 1°C increase in temperature is a protective mechanism.
C) A 5°C increase in temperature is never fatal.
D) None of these are true.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
51
The term "heterogeneous catalyst" means which of the following?
A) a catalyst that does not have uniform composition
B) a catalyst that is of a different phase from the reactants
C) a catalyst that gets consumed completely in the course of the reaction
D) a catalyst that changes its composition in the course of the reaction
A) a catalyst that does not have uniform composition
B) a catalyst that is of a different phase from the reactants
C) a catalyst that gets consumed completely in the course of the reaction
D) a catalyst that changes its composition in the course of the reaction

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
52
A particular chemical reaction carried out at 30°C takes 4 hours. Approximately how long will it take to carry out the reaction at 60°C?
A) 0.5 hour
B) 1 hour
C) 4 hours
D) 8 hours
A) 0.5 hour
B) 1 hour
C) 4 hours
D) 8 hours

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
53
A catalyst speeds up a chemical reaction as a result of which of the following?
A) It increases the number of collisions between reacting molecules.
B) It makes the reaction more endothermic.
C) It makes the reaction more exothermic.
D) none of these
A) It increases the number of collisions between reacting molecules.
B) It makes the reaction more endothermic.
C) It makes the reaction more exothermic.
D) none of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is the best definition of a catalyst?
A) A catalyst is a substance that speeds up the rate of a chemical reaction till its concentration is reduced to zero.
B) A catalyst is a substance that speeds up the rate of a chemical reaction without participating in the reaction.
C) A catalyst is a substance that speeds up the rate of a chemical reaction without being consumed during the reaction.
D) All of these are equally good definitions.
A) A catalyst is a substance that speeds up the rate of a chemical reaction till its concentration is reduced to zero.
B) A catalyst is a substance that speeds up the rate of a chemical reaction without participating in the reaction.
C) A catalyst is a substance that speeds up the rate of a chemical reaction without being consumed during the reaction.
D) All of these are equally good definitions.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
55
The temperature effect on reaction rate is the result of which of the following?
A) At higher temperatures, there are more collisions.
B) At higher temperatures, a larger fraction of the collisions are effective collisions.
C) At higher temperatures, there are more collisions and a larger fraction of the collisions are effective collisions.
D) None of these.
A) At higher temperatures, there are more collisions.
B) At higher temperatures, a larger fraction of the collisions are effective collisions.
C) At higher temperatures, there are more collisions and a larger fraction of the collisions are effective collisions.
D) None of these.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
56
Drug manufacturers use which of the following when making timed-release drugs?
A) control of the thickness of a drug coating
B) control of the size of drugs
C) control of the thickness of a drug coating and the size of drugs
D) none of these
A) control of the thickness of a drug coating
B) control of the size of drugs
C) control of the thickness of a drug coating and the size of drugs
D) none of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is true of a reaction that has reached equilibrium?
A) The reactants have been completely converted to products.
B) The rate of the forward reaction is faster than the rate of the reverse reaction.
C) The rate of the forward reaction is equal to the rate of the reverse reaction.
D) The rate of the forward reaction is slower than the rate of the reverse reaction.
A) The reactants have been completely converted to products.
B) The rate of the forward reaction is faster than the rate of the reverse reaction.
C) The rate of the forward reaction is equal to the rate of the reverse reaction.
D) The rate of the forward reaction is slower than the rate of the reverse reaction.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is true of a reversible reaction?
A) Equilibrium can only be reached by mixing the starting materials.
B) Equilibrium can only be reached by mixing the products.
C) Equilibrium can only be reached by mixing specific amounts of starting materials and products.
D) Equilibrium will always be reached as time progresses.
A) Equilibrium can only be reached by mixing the starting materials.
B) Equilibrium can only be reached by mixing the products.
C) Equilibrium can only be reached by mixing specific amounts of starting materials and products.
D) Equilibrium will always be reached as time progresses.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
59
A particular chemical reaction carried out at 60°C takes 4 hours. Approximately how long will it take to carry out the reaction at 30°C?
A) 0.5 hour
B) 8 hours
C) 4 hours
D) 16 hours
A) 0.5 hour
B) 8 hours
C) 4 hours
D) 16 hours

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
60
What is the purpose of the enteric coating of aspirin?
A) to control time release of the drug in the stomach
B) to ensure drug release before the drug reaches the stomach
C) to ensure drug release in the stomach
D) to ensure drug release in the intestine
A) to control time release of the drug in the stomach
B) to ensure drug release before the drug reaches the stomach
C) to ensure drug release in the stomach
D) to ensure drug release in the intestine

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following are generally included when writing an equilibrium constant expression?
A) masses of pure liquids
B) masses of pure solids
C) concentrations of catalysts in solutions
D) concentrations of pure gases in solutions
A) masses of pure liquids
B) masses of pure solids
C) concentrations of catalysts in solutions
D) concentrations of pure gases in solutions

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
62
Suppose that the equilibrium constant for the chemical reaction is 2.8 x 107. 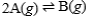 What is the equilibrium constant for the following reaction?
What is the equilibrium constant for the following reaction? 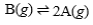
A) 3.6 × 10-8
B) 2.1 × 10-4
C) 4.8 × 103
D) 3.3 × 107
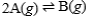 What is the equilibrium constant for the following reaction?
What is the equilibrium constant for the following reaction? 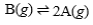
A) 3.6 × 10-8
B) 2.1 × 10-4
C) 4.8 × 103
D) 3.3 × 107

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following will not change the numerical value of the equilibrium constant of a particular reaction?
A) increasing the concentration of reactants
B) increasing the concentration of products
C) increasing the temperature
D) increasing the concentration of reactants and increasing the concentration of products
A) increasing the concentration of reactants
B) increasing the concentration of products
C) increasing the temperature
D) increasing the concentration of reactants and increasing the concentration of products

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
64
For the reaction ![<strong>For the reaction , which of the following is the equilibrium constant expression?</strong> A) K = [H<sub>2</sub>]<sup>2</sup> [O<sub>2</sub>]/[H<sub>2</sub>O] B) K = [H<sub>2</sub>O]<sup>2</sup>/[H<sub>2</sub>]<sup>2</sup> [O<sub>2</sub>] C) K = [H<sub>2</sub>O]<sup>2</sup>/[H<sub>2</sub>][O<sub>2</sub>] D) K = [H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>]/[H<sub>2</sub>O]<sup>2</sup>](https://storage.examlex.com/TB8310/11eb6c44_5842_070a_8d9d_83e3835f63dd_TB8310_11.jpg) , which of the following is the equilibrium constant expression?
, which of the following is the equilibrium constant expression?
A) K = [H2]2 [O2]/[H2O]
B) K = [H2O]2/[H2]2 [O2]
C) K = [H2O]2/[H2][O2]
D) K = [H2]2[O2]/[H2O]2
![<strong>For the reaction , which of the following is the equilibrium constant expression?</strong> A) K = [H<sub>2</sub>]<sup>2</sup> [O<sub>2</sub>]/[H<sub>2</sub>O] B) K = [H<sub>2</sub>O]<sup>2</sup>/[H<sub>2</sub>]<sup>2</sup> [O<sub>2</sub>] C) K = [H<sub>2</sub>O]<sup>2</sup>/[H<sub>2</sub>][O<sub>2</sub>] D) K = [H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>]/[H<sub>2</sub>O]<sup>2</sup>](https://storage.examlex.com/TB8310/11eb6c44_5842_070a_8d9d_83e3835f63dd_TB8310_11.jpg) , which of the following is the equilibrium constant expression?
, which of the following is the equilibrium constant expression?A) K = [H2]2 [O2]/[H2O]
B) K = [H2O]2/[H2]2 [O2]
C) K = [H2O]2/[H2][O2]
D) K = [H2]2[O2]/[H2O]2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
65
In writing the equilibrium constant expression, we use square brackets. What does the notation [A] represent?
A) the mass of A
B) the number of moles of A
C) the molar concentration of A
D) the valency of A
A) the mass of A
B) the number of moles of A
C) the molar concentration of A
D) the valency of A

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
66
For the reaction ![<strong>For the reaction , which of the following is the equilibrium constant expression?</strong> A) K = [H<sub>2</sub>][N<sub>2</sub>]/[NH<sub>3</sub>] B) K = [NH<sub>3</sub>]/[H<sub>2</sub>][N<sub>2</sub>] C) K = [H<sub>2</sub>]<sup>3</sup>[N<sub>2</sub>]/[NH<sub>3</sub>]<sup>2</sup> D) K = [NH<sub>3</sub>]<sup>2</sup>/[H<sub>2</sub>]<sup>3</sup>[N<sub>2</sub>]](https://storage.examlex.com/TB8310/11eb6c44_5842_552b_8d9d_8fcb39096e59_TB8310_11.jpg) , which of the following is the equilibrium constant expression?
, which of the following is the equilibrium constant expression?
A) K = [H2][N2]/[NH3]
B) K = [NH3]/[H2][N2]
C) K = [H2]3[N2]/[NH3]2
D) K = [NH3]2/[H2]3[N2]
![<strong>For the reaction , which of the following is the equilibrium constant expression?</strong> A) K = [H<sub>2</sub>][N<sub>2</sub>]/[NH<sub>3</sub>] B) K = [NH<sub>3</sub>]/[H<sub>2</sub>][N<sub>2</sub>] C) K = [H<sub>2</sub>]<sup>3</sup>[N<sub>2</sub>]/[NH<sub>3</sub>]<sup>2</sup> D) K = [NH<sub>3</sub>]<sup>2</sup>/[H<sub>2</sub>]<sup>3</sup>[N<sub>2</sub>]](https://storage.examlex.com/TB8310/11eb6c44_5842_552b_8d9d_8fcb39096e59_TB8310_11.jpg) , which of the following is the equilibrium constant expression?
, which of the following is the equilibrium constant expression?A) K = [H2][N2]/[NH3]
B) K = [NH3]/[H2][N2]
C) K = [H2]3[N2]/[NH3]2
D) K = [NH3]2/[H2]3[N2]

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following must we know to write the equilibrium constant for a chemical reaction?
A) the balanced chemical equation for the reaction
B) the rate at which the reaction occurs
C) the diameter of the vessel in which the reaction occurs
D) the activation energy of the reaction
A) the balanced chemical equation for the reaction
B) the rate at which the reaction occurs
C) the diameter of the vessel in which the reaction occurs
D) the activation energy of the reaction

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following symbols is used to denote a reversible reaction?
A) →
B) ←
C) ⇌
D) ↔
A) →
B) ←
C) ⇌
D) ↔

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following can be the equilibrium constant for a reaction that does not yield a significant concentration of products?
A) 4 × 10-1
B) 1 × 10-2
C) 1 × 102
D) 1 × 10-15
A) 4 × 10-1
B) 1 × 10-2
C) 1 × 102
D) 1 × 10-15

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following can be the equilibrium constant for a reaction that proceeds very far in the direction of the products?
A) 2 × 10-15
B) 2 × 10-2
C) 2× 102
D) 2 × 1015
A) 2 × 10-15
B) 2 × 10-2
C) 2× 102
D) 2 × 1015

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
71
In an equilibrium constant expression, what does the notation [A]3 represent?
A) [A] + [A] + [A]
B) 1/([A] + [A] + [A])
C) [A] × [A] × [A]
D) 1/([A] × [A] × [A])
A) [A] + [A] + [A]
B) 1/([A] + [A] + [A])
C) [A] × [A] × [A]
D) 1/([A] × [A] × [A])

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
72
For the reaction ![<strong>For the reaction equilibrium is established at 25°C when [NOCl] = 2.6 M, [NO] = 0.34 M, and [Cl<sub>2</sub>] = 1.4 M. What is the equilibrium constant for this reaction?</strong> A) 0.099 B) 0.18 C) 5.4 D) 42.2](https://storage.examlex.com/TB8310/11eb6c44_5844_29f3_8d9d_81fac14d07d4_TB8310_11.jpg) equilibrium is established at 25°C when [NOCl] = 2.6 M, [NO] = 0.34 M, and [Cl2] = 1.4 M. What is the equilibrium constant for this reaction?
equilibrium is established at 25°C when [NOCl] = 2.6 M, [NO] = 0.34 M, and [Cl2] = 1.4 M. What is the equilibrium constant for this reaction?
A) 0.099
B) 0.18
C) 5.4
D) 42.2
![<strong>For the reaction equilibrium is established at 25°C when [NOCl] = 2.6 M, [NO] = 0.34 M, and [Cl<sub>2</sub>] = 1.4 M. What is the equilibrium constant for this reaction?</strong> A) 0.099 B) 0.18 C) 5.4 D) 42.2](https://storage.examlex.com/TB8310/11eb6c44_5844_29f3_8d9d_81fac14d07d4_TB8310_11.jpg) equilibrium is established at 25°C when [NOCl] = 2.6 M, [NO] = 0.34 M, and [Cl2] = 1.4 M. What is the equilibrium constant for this reaction?
equilibrium is established at 25°C when [NOCl] = 2.6 M, [NO] = 0.34 M, and [Cl2] = 1.4 M. What is the equilibrium constant for this reaction?A) 0.099
B) 0.18
C) 5.4
D) 42.2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
73
For the reaction ![<strong>For the reaction equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl<sub>2</sub>] = 0.30 M. What is the equilibrium constant for this reaction?</strong> A) 0.098 B) 0.18 C) 5.4 D) 1.0 × 10<sup>1</sup>](https://storage.examlex.com/TB8310/11eb6c44_5843_b4c2_8d9d_bf6cfa7a74c3_TB8310_11.jpg) equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl2] = 0.30 M. What is the equilibrium constant for this reaction?
equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl2] = 0.30 M. What is the equilibrium constant for this reaction?
A) 0.098
B) 0.18
C) 5.4
D) 1.0 × 101
![<strong>For the reaction equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl<sub>2</sub>] = 0.30 M. What is the equilibrium constant for this reaction?</strong> A) 0.098 B) 0.18 C) 5.4 D) 1.0 × 10<sup>1</sup>](https://storage.examlex.com/TB8310/11eb6c44_5843_b4c2_8d9d_bf6cfa7a74c3_TB8310_11.jpg) equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl2] = 0.30 M. What is the equilibrium constant for this reaction?
equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl2] = 0.30 M. What is the equilibrium constant for this reaction?A) 0.098
B) 0.18
C) 5.4
D) 1.0 × 101

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following will change the numerical value of the equilibrium constant of a particular reaction?
A) increasing the concentration of the products
B) increasing the temperature
C) increasing the concentration of the reactants
D) increasing the concentration of the catalysts
A) increasing the concentration of the products
B) increasing the temperature
C) increasing the concentration of the reactants
D) increasing the concentration of the catalysts

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
75
Suppose the equilibrium constant for the chemical reaction is 3.3 × 107. 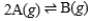 What is the equilibrium constant for the following reaction?
What is the equilibrium constant for the following reaction? 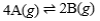
A) 1.99 × 10-5
B) 4.3 × 10-8
C) 3.3 × 107
D) 1.09 × 1015
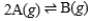 What is the equilibrium constant for the following reaction?
What is the equilibrium constant for the following reaction? 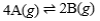
A) 1.99 × 10-5
B) 4.3 × 10-8
C) 3.3 × 107
D) 1.09 × 1015

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
76
Acetic acid is the active ingredient in vinegar. In a solution of acetic acid, the following equilibrium is established. 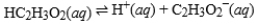 The equilibrium constant for this reaction is 1.5 × 10-5. What is the equilibrium constant for the following reaction?
The equilibrium constant for this reaction is 1.5 × 10-5. What is the equilibrium constant for the following reaction? 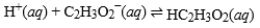
A) 1.8 × 10-5
B) 4.2 × 10-3
C) 6.6 × 104
D) 3.1 × 109
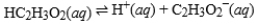 The equilibrium constant for this reaction is 1.5 × 10-5. What is the equilibrium constant for the following reaction?
The equilibrium constant for this reaction is 1.5 × 10-5. What is the equilibrium constant for the following reaction? 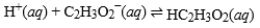
A) 1.8 × 10-5
B) 4.2 × 10-3
C) 6.6 × 104
D) 3.1 × 109

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
77
Consider a sample of water in a closed container. When the physical process 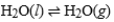 has reached equilibrium, what can we say about any specific water molecule?
has reached equilibrium, what can we say about any specific water molecule?
A) If it was present in the liquid phase, then it will always remain in the liquid phase.
B) If it was present in the vapor phase, then it will always remain in the vapor phase.
C) It will sometimes be in the liquid phase and sometimes be in the vapor phase.
D) If it was present in the liquid phase, then it will always remain in the liquid phase, and if it was present in the vapor phase, then it will always remain in the vapor phase.
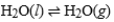 has reached equilibrium, what can we say about any specific water molecule?
has reached equilibrium, what can we say about any specific water molecule?A) If it was present in the liquid phase, then it will always remain in the liquid phase.
B) If it was present in the vapor phase, then it will always remain in the vapor phase.
C) It will sometimes be in the liquid phase and sometimes be in the vapor phase.
D) If it was present in the liquid phase, then it will always remain in the liquid phase, and if it was present in the vapor phase, then it will always remain in the vapor phase.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
78
For the reaction ![<strong>For the reaction equilibrium is established at 472°C when [H<sub>2</sub>] =0.0400 M, [N<sub>2</sub>] = 0.0200 M, and [NH<sub>3</sub>] = 1.074 × 10<sup>-7</sup>M. What is the equilibrium constant for this reaction?</strong> A) 4.69 × 10<sup>-4</sup> B) 9.012 × 10<sup>-9</sup> C) 6.21 × 10<sup>-10</sup> D) 7.41 × 10<sup>-11</sup>](https://storage.examlex.com/TB8310/11eb6c44_5844_7814_8d9d_6b13a59d2496_TB8310_11.jpg) equilibrium is established at 472°C when [H2] =0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-7M. What is the equilibrium constant for this reaction?
equilibrium is established at 472°C when [H2] =0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-7M. What is the equilibrium constant for this reaction?
A) 4.69 × 10-4
B) 9.012 × 10-9
C) 6.21 × 10-10
D) 7.41 × 10-11
![<strong>For the reaction equilibrium is established at 472°C when [H<sub>2</sub>] =0.0400 M, [N<sub>2</sub>] = 0.0200 M, and [NH<sub>3</sub>] = 1.074 × 10<sup>-7</sup>M. What is the equilibrium constant for this reaction?</strong> A) 4.69 × 10<sup>-4</sup> B) 9.012 × 10<sup>-9</sup> C) 6.21 × 10<sup>-10</sup> D) 7.41 × 10<sup>-11</sup>](https://storage.examlex.com/TB8310/11eb6c44_5844_7814_8d9d_6b13a59d2496_TB8310_11.jpg) equilibrium is established at 472°C when [H2] =0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-7M. What is the equilibrium constant for this reaction?
equilibrium is established at 472°C when [H2] =0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-7M. What is the equilibrium constant for this reaction?A) 4.69 × 10-4
B) 9.012 × 10-9
C) 6.21 × 10-10
D) 7.41 × 10-11

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following will change the numerical value of the equilibrium constant of a particular reaction?
A) increasing the concentration of the reactants
B) increasing the concentration of the products
C) increasing the concentration of the reactants and the products
D) none of these
A) increasing the concentration of the reactants
B) increasing the concentration of the products
C) increasing the concentration of the reactants and the products
D) none of these

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
80
For the reaction ![<strong>For the reaction equilibrium is established at 472°C when [H<sub>2</sub>] = 0.0400 M, [N<sub>2</sub>] = 0.0200 M, and [NH<sub>3</sub>] = 1.074 × 10<sup>-8</sup>M. What is the equilibrium constant for the reverse reaction?</strong> A) 3.72 × 10<sup>3</sup> B) 1.109 × 10<sup>11</sup> C) 1.39 × 10<sup>9</sup> D) 6.94 × 10<sup>10</sup>](https://storage.examlex.com/TB8310/11eb6c44_5844_c635_8d9d_ffb44077c0b9_TB8310_11.jpg) equilibrium is established at 472°C when [H2] = 0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-8M. What is the equilibrium constant for the reverse reaction?
equilibrium is established at 472°C when [H2] = 0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-8M. What is the equilibrium constant for the reverse reaction?
A) 3.72 × 103
B) 1.109 × 1011
C) 1.39 × 109
D) 6.94 × 1010
![<strong>For the reaction equilibrium is established at 472°C when [H<sub>2</sub>] = 0.0400 M, [N<sub>2</sub>] = 0.0200 M, and [NH<sub>3</sub>] = 1.074 × 10<sup>-8</sup>M. What is the equilibrium constant for the reverse reaction?</strong> A) 3.72 × 10<sup>3</sup> B) 1.109 × 10<sup>11</sup> C) 1.39 × 10<sup>9</sup> D) 6.94 × 10<sup>10</sup>](https://storage.examlex.com/TB8310/11eb6c44_5844_c635_8d9d_ffb44077c0b9_TB8310_11.jpg) equilibrium is established at 472°C when [H2] = 0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-8M. What is the equilibrium constant for the reverse reaction?
equilibrium is established at 472°C when [H2] = 0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-8M. What is the equilibrium constant for the reverse reaction?A) 3.72 × 103
B) 1.109 × 1011
C) 1.39 × 109
D) 6.94 × 1010

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck


