Exam 7: Reaction Rates and Chemical Equilibrium
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
In the reaction of HCl with water: 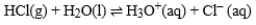 The following species forms.
The following species forms. 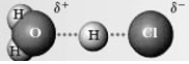 In the given energy diagram, which letter represents this species?
In the given energy diagram, which letter represents this species?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
C
Which of the following can be the equilibrium constant for a reaction that proceeds very far in the direction of the products?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
For the reaction ![For the reaction equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl<sub>2</sub>] = 0.30 M. What is the equilibrium constant for this reaction?](https://storage.examlex.com/TB8310/11eb6c44_5843_b4c2_8d9d_bf6cfa7a74c3_TB8310_11.jpg) equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl2] = 0.30 M. What is the equilibrium constant for this reaction?
equilibrium is established at 25°C when [NOCl] = 2.1 M, [NO] = 1.2 M, and [Cl2] = 0.30 M. What is the equilibrium constant for this reaction?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
A
For the reaction 2C → A + B, 0.02 mol of A is formed during the first 15 seconds of the reaction. Assuming that the rate of reaction remains constant for 2 min, which of the following statements is true?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following will change the numerical value of the equilibrium constant of a particular reaction?
(Multiple Choice)
5.0/5  (26)
(26)
How does the rate of reaction change when the reactant is used up over the course of a reaction?
(Multiple Choice)
4.9/5  (31)
(31)
What is the effect of adding CH3COOC2H5(g)to a container in which the following reaction has reached equilibrium? 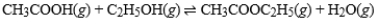
(Multiple Choice)
5.0/5  (28)
(28)
Which of the following does not result from increasing the temperature of a reaction?
(Multiple Choice)
4.7/5  (44)
(44)
In an energy diagram for an endothermic chemical reaction, which of the following is true?
(Multiple Choice)
4.9/5  (35)
(35)
In an equilibrium constant expression, what does the notation [A]3 represent?
(Multiple Choice)
4.8/5  (50)
(50)
For the reaction ![For the reaction equilibrium is established at 472°C when [H<sub>2</sub>] = 0.0400 M, [N<sub>2</sub>] = 0.0200 M, and [NH<sub>3</sub>] = 1.074 × 10<sup>-8</sup>M. What is the equilibrium constant for the reverse reaction?](https://storage.examlex.com/TB8310/11eb6c44_5844_c635_8d9d_ffb44077c0b9_TB8310_11.jpg) equilibrium is established at 472°C when [H2] = 0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-8M. What is the equilibrium constant for the reverse reaction?
equilibrium is established at 472°C when [H2] = 0.0400 M, [N2] = 0.0200 M, and [NH3] = 1.074 × 10-8M. What is the equilibrium constant for the reverse reaction?
(Multiple Choice)
4.9/5  (43)
(43)
Consider the following energy diagram for a reaction. 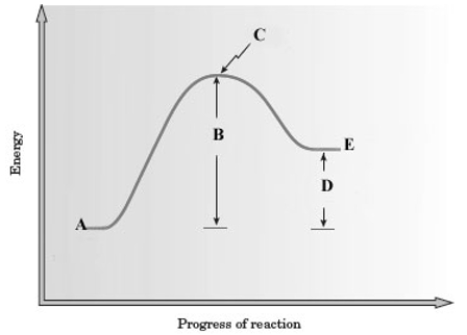 Which letter represents the net energy change of the reaction?
Which letter represents the net energy change of the reaction?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following is true of the rates of most chemical reactions?
(Multiple Choice)
4.8/5  (41)
(41)
Consider a sample of water in a closed, rigid container. Which of the following will indicate that the given physical process has reached equilibrium? 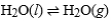
(Multiple Choice)
4.8/5  (28)
(28)
A particular reaction has an equilibrium constant of 1 x 10-15. Which of the following best describes the relationship between the equilibrium constant and the reaction rate?
(Multiple Choice)
4.8/5  (36)
(36)
If a chemist wishes to react a solid with a liquid, which of the following will be the least effective in speeding up the reaction?
(Multiple Choice)
4.9/5  (28)
(28)
If a chemist wishes to react a solid with a liquid, which of the following will be the most effective in speeding up the reaction?
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following will change the numerical value of the equilibrium constant of a particular reaction?
(Multiple Choice)
4.8/5  (45)
(45)
Why will increasing the temperature of a reaction speed up the reaction?
(Multiple Choice)
4.9/5  (35)
(35)
Consider the following energy diagram for a reaction. 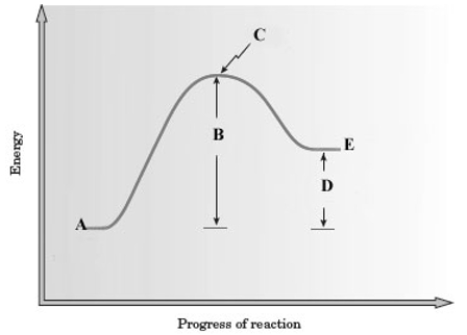 Which of the following is a correct interpretation of the graph?
Which of the following is a correct interpretation of the graph?
(Multiple Choice)
4.9/5  (29)
(29)
Showing 1 - 20 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)