Deck 12: Alkenes, Alkynes, and Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/186
Play
Full screen (f)
Deck 12: Alkenes, Alkynes, and Aromatic Compounds
1
Given an alkane, an alkene, and an alkyne, each of which contains two carbon atoms, which compound will contain the fewest number of hydrogen atoms?
A) the alkane
B) the alkene
C) the alkyne
D) none of these
A) the alkane
B) the alkene
C) the alkyne
D) none of these
C
2
Which of the following characteristics is associated with the presence of cis-trans isomerism?
A) the presence of double bonds
B) the presence of ring systems
C) the presence of both double bonds and ring systems
D) the absence of both double bonds and ring systems
A) the presence of double bonds
B) the presence of ring systems
C) the presence of both double bonds and ring systems
D) the absence of both double bonds and ring systems
C
3
Which of the following is true of ethylene?
A) It is not an important industrial chemical.
B) It is present in large amounts in natural gas.
C) It is formed by the catalytic hydrogenation of ethane.
D) It is produced by heating ethane to around 800-900°C.
A) It is not an important industrial chemical.
B) It is present in large amounts in natural gas.
C) It is formed by the catalytic hydrogenation of ethane.
D) It is produced by heating ethane to around 800-900°C.
D
4
Which of the following is true of the IUPAC system of naming alkenes?
A) The alkene is always named based on the shortest carbon chain in a branched structure.
B) The position of the double bond is always given the highest possible number.
C) The position and identity of substituents determine the number associated with the position of the double bond.
D) None of these are correct.
A) The alkene is always named based on the shortest carbon chain in a branched structure.
B) The position of the double bond is always given the highest possible number.
C) The position and identity of substituents determine the number associated with the position of the double bond.
D) None of these are correct.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
5
What is the observed H-C-C bond angle in ethylene?
A) 180°
B) 90°
C) 109.5°
D) 121.7°
A) 180°
B) 90°
C) 109.5°
D) 121.7°

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
6
Given an alkane, an alkene, and an alkyne, each of which contains two carbon atoms, which compound will contain the greatest number of hydrogen atoms?
A) the alkane
B) the alkene
C) the alkyne
D) none of these
A) the alkane
B) the alkene
C) the alkyne
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
7
Which class of compounds are not widespread in nature and have little importance in biochemistry?
A) alkanes
B) alkenes
C) alkynes
D) arenes
A) alkanes
B) alkenes
C) alkynes
D) arenes

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
8
In alkenes, there are four groups bonded to the carbons that constitute the carbon-carbon double bond. Which of the following situations produces cis-trans isomerism?
A) when all four groups are the same
B) when the two groups bonded to each carbon atom are different from one another
C) when three of the four groups in an alkene are the same, but the fourth group is different
D) none of these
A) when all four groups are the same
B) when the two groups bonded to each carbon atom are different from one another
C) when three of the four groups in an alkene are the same, but the fourth group is different
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following structural features is never associated with cis-trans isomerism?
A) double bonds
B) ring systems
C) triple bonds
D) none of these
A) double bonds
B) ring systems
C) triple bonds
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
10
In alkenes, there are four groups bonded to the carbons that constitute the carbon-carbon double bond. Which of the following situations produces cis-trans isomerism?
A) when all four groups are the same
B) when one double-bonded carbon is bonded to two identical groups and the other double-bonded carbon is bonded to two other identical groups
C) when three of the four groups are the same, but the fourth group is different
D) none of these
A) when all four groups are the same
B) when one double-bonded carbon is bonded to two identical groups and the other double-bonded carbon is bonded to two other identical groups
C) when three of the four groups are the same, but the fourth group is different
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is true of the IUPAC system of naming alkenes?
A) The alkene is named based on the longest carbon chain that contains the double bond.
B) The position of the double bond is always given the highest possible number.
C) The position and identity of substituents determine the number associated with the position of the double bond.
D) None of these are correct.
A) The alkene is named based on the longest carbon chain that contains the double bond.
B) The position of the double bond is always given the highest possible number.
C) The position and identity of substituents determine the number associated with the position of the double bond.
D) None of these are correct.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
12
In which of the following hydrocarbons is the bond angle about the carbon atom approximately 120°?
A) alkanes
B) alkenes
C) alkynes
D) none of these
A) alkanes
B) alkenes
C) alkynes
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
13
Which compound derived from natural gas is a starting material for the production of ethylene?
A) acetylene
B) benzene
C) ethane
D) methane
A) acetylene
B) benzene
C) ethane
D) methane

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is true of the six atoms in ethylene?
A) The hydrogens on either side of the carbon-carbon double bond lie in different planes.
B) The carbon and hydrogen atoms lie in the same plane.
C) The carbon and hydrogen atoms bond to form a tetrahedral molecule.
D) The two carbon atoms lie in one plane, and the four hydrogen atoms lie in another plane.
A) The hydrogens on either side of the carbon-carbon double bond lie in different planes.
B) The carbon and hydrogen atoms lie in the same plane.
C) The carbon and hydrogen atoms bond to form a tetrahedral molecule.
D) The two carbon atoms lie in one plane, and the four hydrogen atoms lie in another plane.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following compounds have cis-trans isomers because they lack free rotation about their carbon-carbon bonds?
A) alkanes
B) alkenes
C) alkynes
D) haloalkanes
A) alkanes
B) alkenes
C) alkynes
D) haloalkanes

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is true of cis and trans isomers?
A) They have the same structural connectivity.
B) They have the same physical properties.
C) They have the same structural connectivity and the same physical properties.
D) None of these.
A) They have the same structural connectivity.
B) They have the same physical properties.
C) They have the same structural connectivity and the same physical properties.
D) None of these.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a characteristic of alkenes?
A) the presence of one or more carbon-carbon double bonds
B) the presence of one or more carbon-carbon triple bonds
C) the presence of a hydroxyl group
D) the presence of a ring system
A) the presence of one or more carbon-carbon double bonds
B) the presence of one or more carbon-carbon triple bonds
C) the presence of a hydroxyl group
D) the presence of a ring system

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the most common method used to manufacture ethylene?
A) the polymerization of hydrocarbons
B) the thermal cracking of hydrocarbons
C) the condensation of hydrocarbons
D) the biological degradation of hydrocarbons
A) the polymerization of hydrocarbons
B) the thermal cracking of hydrocarbons
C) the condensation of hydrocarbons
D) the biological degradation of hydrocarbons

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a characteristic of alkynes?
A) the presence of one or more carbon-carbon double bonds
B) the presence of one or more carbon-carbon triple bonds
C) the presence of cis-trans isomers
D) the presence of a ring system
A) the presence of one or more carbon-carbon double bonds
B) the presence of one or more carbon-carbon triple bonds
C) the presence of cis-trans isomers
D) the presence of a ring system

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
20
To which class of compounds do alkenes and alkynes containing only carbon and hydrogen belong?
A) halogenated compounds
B) alcoholic compounds
C) aromatic compounds
D) unsaturated compounds
A) halogenated compounds
B) alcoholic compounds
C) aromatic compounds
D) unsaturated compounds

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
21
What is the correct IUPAC name for the following compound? 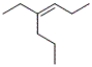
A) trans-3-propyl-3-hexene
B) cis-3-propyl-3-hexene
C) trans-4-ethyl-3-heptene
D) cis-4-ethyl-3-heptene

A) trans-3-propyl-3-hexene
B) cis-3-propyl-3-hexene
C) trans-4-ethyl-3-heptene
D) cis-4-ethyl-3-heptene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds with the molecular formula C5H10 has cis and trans isomers?
A) 2-methyl-1-butene
B) 3-methyl-1-butene
C) 2-pentene
D) 1-pentene
A) 2-methyl-1-butene
B) 3-methyl-1-butene
C) 2-pentene
D) 1-pentene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is not a correct IUPAC name for an alkene?
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 3-methyl-1-butene
D) None, these are all correct IUPAC names.
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 3-methyl-1-butene
D) None, these are all correct IUPAC names.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
24
What is the correct IUPAC name for the following compound? 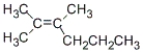
A) 2-methyl-3-propyl-2-butene
B) 1,1,2-trimethyl-1-pentene
C) 2,3-dimethyl-2-hexene
D) 4,5-dimethyl-4-hexene

A) 2-methyl-3-propyl-2-butene
B) 1,1,2-trimethyl-1-pentene
C) 2,3-dimethyl-2-hexene
D) 4,5-dimethyl-4-hexene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
25
Cis-trans isomers exist for which of the following molecules, all of which have the molecular formula C5H10?
A) 1-pentene
B) 2-methyl-1-butene
C) 3-methyl-1-butene
D) 1,2-dimethylcyclopropane
A) 1-pentene
B) 2-methyl-1-butene
C) 3-methyl-1-butene
D) 1,2-dimethylcyclopropane

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
26
A student wanted to use the branched groups, isobutyl, sec-butyl, and tert-butyl when naming alkenes but was told not to. What is the correct IUPAC name for the compound the student called sec-butylethene?
A) 4-methyl-1-pentene
B) 3-methyl-1-pentene
C) 3,3-dimethyl-1-butene
D) none of these
A) 4-methyl-1-pentene
B) 3-methyl-1-pentene
C) 3,3-dimethyl-1-butene
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is true of the IUPAC system of naming alkenes?
A) The alkene is always named based on the shortest carbon chain in a branched structure.
B) The position of the double bond is always given the lowest possible number.
C) The position and identity of substituents determine the number associated with the position of the double bond.
D) None of these are correct.
A) The alkene is always named based on the shortest carbon chain in a branched structure.
B) The position of the double bond is always given the lowest possible number.
C) The position and identity of substituents determine the number associated with the position of the double bond.
D) None of these are correct.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
28
What is the name of the lightest hydrocarbon that has cis and trans isomers?
A) 1-butene
B) 2-butene
C) 1,2-dimethylcyclopropane
D) propene
A) 1-butene
B) 2-butene
C) 1,2-dimethylcyclopropane
D) propene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is true of ethylene and acetylene?
A) Ethylene contains more carbon atoms than acetylene does.
B) Ethylene has a carbon-carbon double bond, and acetylene has a carbon-carbon triple bond.
C) Ethylene and acetylene are the IUPAC names of two-carbon compounds.
D) Ethylene and acetylene belong to a single class of compounds called alkenes.
A) Ethylene contains more carbon atoms than acetylene does.
B) Ethylene has a carbon-carbon double bond, and acetylene has a carbon-carbon triple bond.
C) Ethylene and acetylene are the IUPAC names of two-carbon compounds.
D) Ethylene and acetylene belong to a single class of compounds called alkenes.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
30
Cis-trans isomers do not exist for which of the following compounds, all of which have the molecular formula C3H4Br2?
A) 1,1-dibromopropene
B) 1,2-dibromopropene
C) 1,3-dibromopropene
D) none of these
A) 1,1-dibromopropene
B) 1,2-dibromopropene
C) 1,3-dibromopropene
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
31
A student named a particular compound 2-ethyl-3-methyl-2-butene. Assuming that the student's choice actually corresponded to the correct distribution of the double bond and the substituents, what is the correct IUPAC name for this compound?
A) 2-ethyl-3-methyl-2-butene
B) 3,4-dimethyl-3-pentene
C) 2,3-dimethyl-2-pentene
D) 2,3-dimethyl-1-pentene
A) 2-ethyl-3-methyl-2-butene
B) 3,4-dimethyl-3-pentene
C) 2,3-dimethyl-2-pentene
D) 2,3-dimethyl-1-pentene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is not a correct IUPAC name for an alkene?
A) 1-methyl-1-butene
B) 2-methyl-1-butene
C) 3-methyl-1-butene
D) 2-methyl-2-butene
A) 1-methyl-1-butene
B) 2-methyl-1-butene
C) 3-methyl-1-butene
D) 2-methyl-2-butene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is not a correct IUPAC name for an alkene?
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 2-methyl-3-butene
D) None, these are all correct IUPAC names.
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 2-methyl-3-butene
D) None, these are all correct IUPAC names.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
34
Cis-trans isomers exist for which of the following molecules, all of which have the molecular formula C3H5Br?
A) 1-bromopropene
B) 2-bromopropene
C) 3-bromopropene
D) none of these
A) 1-bromopropene
B) 2-bromopropene
C) 3-bromopropene
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following compounds with the molecular formula C6H12 does not have cis and trans isomers?
A) 4-methyl-2-pentene
B) 2-methyl-1-pentene
C) 3-methyl-2-pentene
D) None, they all have cis isomers.
A) 4-methyl-2-pentene
B) 2-methyl-1-pentene
C) 3-methyl-2-pentene
D) None, they all have cis isomers.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
36
A student wanted to use the branched groups, isobutyl, sec-butyl, and tert-butyl when naming alkenes but was told not to. What is the correct IUPAC name for the compound the student called isobutylethene?
A) 4-methyl-1-pentene
B) 3-methyl-1-pentene
C) 3,3-dimethyl-1-butene
D) none of these
A) 4-methyl-1-pentene
B) 3-methyl-1-pentene
C) 3,3-dimethyl-1-butene
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following compounds with the molecular formula C6H12 has cis and trans isomers?
A) 2-methyl-1-pentene
B) 2-methyl-2-pentene
C) 3-methyl-2-pentene
D) cyclohexane
A) 2-methyl-1-pentene
B) 2-methyl-2-pentene
C) 3-methyl-2-pentene
D) cyclohexane

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following structures corresponds to cis-3-methyl-2-hexene?
A)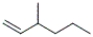
B)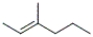
C)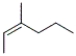
D)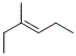
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
39
A student named a particular compound 2-propyl-1-butene. Assuming that the student's choice actually corresponded to the correct distribution of the double bond and the substituents, what is the correct IUPAC name for this compound?
A) 2-propyl-1-butene
B) 2-ethyl-1-pentene
C) 3-methyl-2-pentene
D) none of these
A) 2-propyl-1-butene
B) 2-ethyl-1-pentene
C) 3-methyl-2-pentene
D) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
40
What is the correct IUPAC name for the following compound? 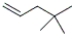
A) 2,2-dimethyl-4-pentene
B) 4,4-dimethyl-2-pentene
C) 4,4-dimethyl-1-pentene
D) isoheptene

A) 2,2-dimethyl-4-pentene
B) 4,4-dimethyl-2-pentene
C) 4,4-dimethyl-1-pentene
D) isoheptene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
41
What is the configuration of each of the given alkenes? 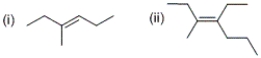
A) (i) cis (ii) cis
B) (i) trans (ii) trans
C) (i) cis (ii) trans
D) (i) trans (ii) cis
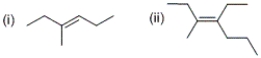
A) (i) cis (ii) cis
B) (i) trans (ii) trans
C) (i) cis (ii) trans
D) (i) trans (ii) cis

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
42
What is the IUPAC name of the following molecule? 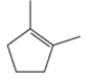
A) 2,2-diethylcyclopentene
B) 1,1-dimethylcyclopentene
C) 1,2-dimethylcyclopentene
D) 1,3-dimethylcyclopentene

A) 2,2-diethylcyclopentene
B) 1,1-dimethylcyclopentene
C) 1,2-dimethylcyclopentene
D) 1,3-dimethylcyclopentene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
43
Including cis-trans isomers, how many stereoisomers are possible for an alkene that has the molecular formula C4H8?
A) 2
B) 6
C) 4
D) 5
A) 2
B) 6
C) 4
D) 5

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following attractive forces are found among the molecules of alkenes and alkynes?
A) electromagnetic forces
B) London dispersion forces
C) nuclear forces
D) strong ionic forces
A) electromagnetic forces
B) London dispersion forces
C) nuclear forces
D) strong ionic forces

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
45
In which of the following compounds is free rotation about all bonds possible?
A) 2-butene
B) cyclobutane
C) butane
D) butyne
A) 2-butene
B) cyclobutane
C) butane
D) butyne

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following reagents is used to convert cyclohexene to 1-iodo-cyclohexane?
A) hydrogen peroxide
B) iodine in dichloromethane
C) hydrogen iodide
D) nitric acid in sulfuric acid
A) hydrogen peroxide
B) iodine in dichloromethane
C) hydrogen iodide
D) nitric acid in sulfuric acid

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is not a correct IUPAC name?
A) cis-2,3-dimethylcyclopentene
B) cis-3,4-dimethylcyclopentene
C) cis-3,5-dimethylcyclopentene
D) trans-3,4-dimethylcyclopentene
A) cis-2,3-dimethylcyclopentene
B) cis-3,4-dimethylcyclopentene
C) cis-3,5-dimethylcyclopentene
D) trans-3,4-dimethylcyclopentene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is true of the noncyclic portion of vitamin A?
A) It contains 4 double bonds, all in the cis configuration.
B) It contains 4 double bonds, all in the trans configuration.
C) It contains 4 double bonds that alternate as cis, trans, cis, and trans.
D) It contains 4 double bonds that alternate as trans, cis, trans, and cis.
A) It contains 4 double bonds, all in the cis configuration.
B) It contains 4 double bonds, all in the trans configuration.
C) It contains 4 double bonds that alternate as cis, trans, cis, and trans.
D) It contains 4 double bonds that alternate as trans, cis, trans, and cis.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is true of 2-methyl-1,3-butadiene?
A) It contains two double bonds adjacent to each other.
B) It contains four cis-trans isomers.
C) It does not exhibit cis-trans isomerism.
D) It contains a C-C-C bond angle equal to 109°.
A) It contains two double bonds adjacent to each other.
B) It contains four cis-trans isomers.
C) It does not exhibit cis-trans isomerism.
D) It contains a C-C-C bond angle equal to 109°.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following strategies is used while naming a cycloalkene?
A) The substituents are numbered first, and the numbering is continued in the direction where the double bond gets the lowest possible number.
B) The cyclic ring is broken down at one of the positions of the double bond, and the numbering is done as with the alkenes.
C) The double bond is numbered 1 and 2 in the direction where a substituent gets the lowest possible number.
D) The double bond is numbered 1 and 2 in the direction where a substituent gets the highest possible number.
A) The substituents are numbered first, and the numbering is continued in the direction where the double bond gets the lowest possible number.
B) The cyclic ring is broken down at one of the positions of the double bond, and the numbering is done as with the alkenes.
C) The double bond is numbered 1 and 2 in the direction where a substituent gets the lowest possible number.
D) The double bond is numbered 1 and 2 in the direction where a substituent gets the highest possible number.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
51
How many stereoisomers are possible for 1,4,6-octatriene?
A) 2
B) 4
C) 8
D) 16
A) 2
B) 4
C) 8
D) 16

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the similarity between the following two molecules. 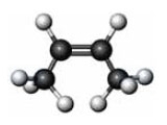
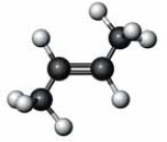
A) Both are cis isomers.
B) Both have the same boiling point.
C) Both have the same melting point.
D) Both undergo hydrogenation reactions.


A) Both are cis isomers.
B) Both have the same boiling point.
C) Both have the same melting point.
D) Both undergo hydrogenation reactions.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
53
Including cis-trans isomers, how many different stereoisomers are possible for an alkene that has the molecular formula C5H10?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
54
How many stereoisomers are possible for 2,4-heptadiene?
A) 2
B) 4
C) 7
D) 8
A) 2
B) 4
C) 7
D) 8

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following molecules with the molecular formula C5H8 has stereoisomers?
A) 1,3-pentadiene
B) 1,4-pentadiene
C) 2-methyl-1,3-butadiene
D) 3-methyl-1,3-butadiene
A) 1,3-pentadiene
B) 1,4-pentadiene
C) 2-methyl-1,3-butadiene
D) 3-methyl-1,3-butadiene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
56
Why is the C-C-C bond angle in propene 124.7° instead of the predicted angle of 120°?
A) because of the presence of a tetrahedral structure
B) because of the presence of two trans methyl groups
C) because of the interaction between the methyl group and the double-bonded carbon
D) because of free rotation about the double-bonded carbons
A) because of the presence of a tetrahedral structure
B) because of the presence of two trans methyl groups
C) because of the interaction between the methyl group and the double-bonded carbon
D) because of free rotation about the double-bonded carbons

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is not a correct IUPAC name?
A) 3-methylcyclopentene
B) 4-methylcyclopentene
C) 5-methylcyclopentene
D) 1-methylcyclopentene
A) 3-methylcyclopentene
B) 4-methylcyclopentene
C) 5-methylcyclopentene
D) 1-methylcyclopentene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
58
How many stereoisomers are possible for 1,4,7-octatriene?
A) 2
B) 4
C) 8
D) 16
A) 2
B) 4
C) 8
D) 16

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is a correct IUPAC name?
A) 4-ethyl-1-methylcyclohexene
B) 5-ethyl-2-methylcyclohexene
C) 1-methyl-4-ethylcyclohexene
D) 5-methyl-2-ethylcyclohexene
A) 4-ethyl-1-methylcyclohexene
B) 5-ethyl-2-methylcyclohexene
C) 1-methyl-4-ethylcyclohexene
D) 5-methyl-2-ethylcyclohexene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
60
How many stereoisomers are possible for 1,4-heptadiene?
A) 2
B) 4
C) 7
D) 8
A) 2
B) 4
C) 7
D) 8

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following reactions involves the addition of two equivalent groups to both the ends of a double bond?
A) bromination
B) hydrogenation
C) both bromination and hydrogenation
D) neither bromination nor hydrogenation
A) bromination
B) hydrogenation
C) both bromination and hydrogenation
D) neither bromination nor hydrogenation

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following statements is true of an electrophile?
A) It is an electron-rich species.
B) It reacts with an electron-donor species to form a new bond.
C) It attacks an electron-deficient species.
D) It is a stable species and does not undergo any reaction.
A) It is an electron-rich species.
B) It reacts with an electron-donor species to form a new bond.
C) It attacks an electron-deficient species.
D) It is a stable species and does not undergo any reaction.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following reactions involves the addition of different groups to both the ends of a double bond?
A) hydration
B) hydrogenation
C) both hydration and hydrogenation
D) neither hydration nor hydrogenation
A) hydration
B) hydrogenation
C) both hydration and hydrogenation
D) neither hydration nor hydrogenation

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the order of reactions in the mechanism of an addition reaction between HCl and 2-butene.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following products is formed when the given substance reacts with hydrogen? 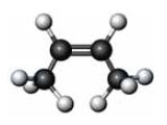
A) butyne
B) cis-butane
C) trans-butene
D) butane

A) butyne
B) cis-butane
C) trans-butene
D) butane

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following reactions involves the addition of different groups to both the ends of a double bond?
A) hydrogenation
B) hydrobromination
C) both hydrogenation and hydrobromination
D) neither hydrogenation nor hydrobromiantion
A) hydrogenation
B) hydrobromination
C) both hydrogenation and hydrobromination
D) neither hydrogenation nor hydrobromiantion

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
67
What happens when the following substance reacts with water in the presence of concentrated sulfuric acid? 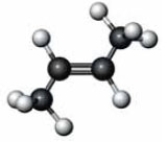
A) An alkyne is produced.
B) An alcohol is produced.
C) The substance is converted to its cis isomer.
D) The substance is converted to its trans isomer.

A) An alkyne is produced.
B) An alcohol is produced.
C) The substance is converted to its cis isomer.
D) The substance is converted to its trans isomer.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is the most characteristic reaction of alkenes?
A) addition
B) oxidation
C) reduction
D) substitution
A) addition
B) oxidation
C) reduction
D) substitution

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following curved arrows correctly describes the second step in the reaction of propene with hydrogen bromide?
A)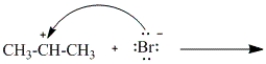
B)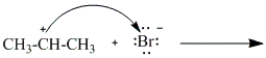
C)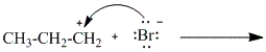
D)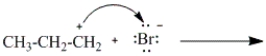
A)
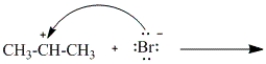
B)

C)
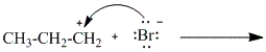
D)
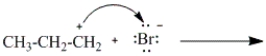

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements is true of the solubility of alkenes?
A) All alkenes are soluble in water.
B) Long chain alkenes are soluble in water, but short chain alkenes are insoluble.
C) Long chain alkenes are insoluble in water, but short chain alkenes are soluble.
D) All alkenes are soluble in alkanes.
A) All alkenes are soluble in water.
B) Long chain alkenes are soluble in water, but short chain alkenes are insoluble.
C) Long chain alkenes are insoluble in water, but short chain alkenes are soluble.
D) All alkenes are soluble in alkanes.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the alkyne counterpart of the following compound. 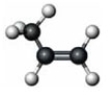 Which of the following would characterize this alkyne?
Which of the following would characterize this alkyne?
A) C4H10
B)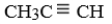
C)
D) C2H2
E) none of these
 Which of the following would characterize this alkyne?
Which of the following would characterize this alkyne?A) C4H10
B)
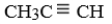
C)

D) C2H2
E) none of these

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
72
Examine the following reaction. 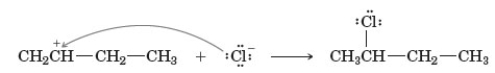 Which of the following describes this reaction?
Which of the following describes this reaction?
A) It involves a secondary carbocation.
B) A represents the nucleophile.
C) B represents the electrophile.
D) It involves the formation of a new carbon-carbon bond.
E) All of these are correct.
 Which of the following describes this reaction?
Which of the following describes this reaction?A) It involves a secondary carbocation.
B) A represents the nucleophile.
C) B represents the electrophile.
D) It involves the formation of a new carbon-carbon bond.
E) All of these are correct.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the substance represented by the following model. 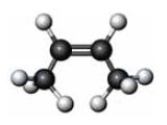 The physical properties of this substance would most closely represent the corresponding member of which functional group class?
The physical properties of this substance would most closely represent the corresponding member of which functional group class?
A) an alkyne
B) an alcohol
C) an aldehyde
D) a carboxylic acid
 The physical properties of this substance would most closely represent the corresponding member of which functional group class?
The physical properties of this substance would most closely represent the corresponding member of which functional group class?A) an alkyne
B) an alcohol
C) an aldehyde
D) a carboxylic acid

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the given molecule. 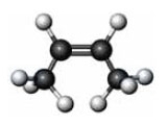 Which of the following is a characteristic of the given molecule?
Which of the following is a characteristic of the given molecule?
A) It is an alkene.
B) It is a cis isomer.
C) It is the stereoisomer of the following molecule.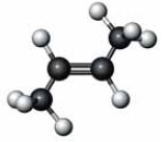
D) All of these are characteristics of this compound.
 Which of the following is a characteristic of the given molecule?
Which of the following is a characteristic of the given molecule?A) It is an alkene.
B) It is a cis isomer.
C) It is the stereoisomer of the following molecule.

D) All of these are characteristics of this compound.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
75
Markovnikov's rule is not useful for predicting the outcome of the reaction of hydrogen chloride with which of the following alkenes?
A) 1-butene
B) 2-butene
C) 2-methylpropene
D) 1-pentene
A) 1-butene
B) 2-butene
C) 2-methylpropene
D) 1-pentene

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is true of the given compound? 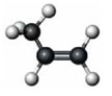
A) It is the simplest alkene.
B) It is an alkyne.
C) It has at least one bond angle close to 120°.
D) It has free rotation about all its bonds.

A) It is the simplest alkene.
B) It is an alkyne.
C) It has at least one bond angle close to 120°.
D) It has free rotation about all its bonds.

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
77
addition of a proton to 2-butene
A) 1, 2, 3
B) 2, 1, 3
C) 3, 2, 1
D) 2, 3, 1
A) 1, 2, 3
B) 2, 1, 3
C) 3, 2, 1
D) 2, 3, 1

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following reactions involves the addition of different groups to both the ends of a double bond?
A) bromination
B) hydrogenation
C) both bromination and hydrogenation
D) neither bromination nor hydrogenation
A) bromination
B) hydrogenation
C) both bromination and hydrogenation
D) neither bromination nor hydrogenation

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
79
Propene reacts with hydrogen chloride to form mostly 2-chloropropane. This reaction does not lead to the formation of 1-chloropropane. Why?
A) because it is a substitution reaction
B) because it is a hydrogenation reaction
C) because it is a regioselective reaction
D) because it is a hydration reaction
A) because it is a substitution reaction
B) because it is a hydrogenation reaction
C) because it is a regioselective reaction
D) because it is a hydration reaction

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck
80
reaction of a chloride ion with an intermediate to form 2-chlorobutane

Unlock Deck
Unlock for access to all 186 flashcards in this deck.
Unlock Deck
k this deck


