Exam 12: Alkenes, Alkynes, and Aromatic Compounds
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
Consider the following structure. 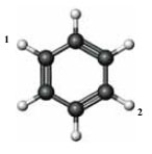 If a chlorine atom is substituted for each of the labeled hydrogen atoms, which of the following is true of the resulting compound?
If a chlorine atom is substituted for each of the labeled hydrogen atoms, which of the following is true of the resulting compound?
Free
(Multiple Choice)
4.8/5  (47)
(47)
Correct Answer:
C
Which type of intermediate is generated during the acid-catalyzed hydration of an alkene?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
C
Including cis-trans isomers, how many stereoisomers are possible for an alkene that has the molecular formula C4H8?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following characteristics is associated with the presence of cis-trans isomerism?
(Multiple Choice)
4.7/5  (21)
(21)
A student named a particular compound 2-propyl-1-butene. Assuming that the student's choice actually corresponded to the correct distribution of the double bond and the substituents, what is the correct IUPAC name for this compound?
(Multiple Choice)
4.8/5  (29)
(29)
What happens when the following substance reacts with water in the presence of concentrated sulfuric acid? 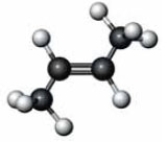
(Multiple Choice)
4.7/5  (34)
(34)
In which of the following hydrocarbons is the bond angle about the carbon atom approximately 120°?
(Multiple Choice)
4.8/5  (22)
(22)
Which of the following observations was troublesome to 19th-century chemists in the determination of benzene's structure?
(Multiple Choice)
4.8/5  (47)
(47)
Consider the substance represented by the following model. 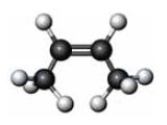 The physical properties of this substance would most closely represent the corresponding member of which functional group class?
The physical properties of this substance would most closely represent the corresponding member of which functional group class?
(Multiple Choice)
4.9/5  (43)
(43)
Which product is formed by the chlorination of 2-methyl-1-butene?
(Multiple Choice)
4.8/5  (38)
(38)
Examine the following reaction. 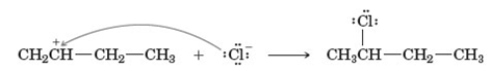 This reaction occurs in which step of the hydrohalogenation reaction mechanism?
This reaction occurs in which step of the hydrohalogenation reaction mechanism?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following catalysts are used for producing low-density polyethylene from ethylene?
(Multiple Choice)
4.8/5  (32)
(32)
How should a benzene ring with a methyl substituent and a hydroxyl substituent be named according to IUPAC nomenclature?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following reactions requires a transition metal catalyst?
(Multiple Choice)
4.9/5  (38)
(38)
What is the charge on the organic intermediate formed during hydrohalogenation?
(Multiple Choice)
4.9/5  (48)
(48)
Which of the following reactions involves the addition of different groups to both the ends of a double bond?
(Multiple Choice)
4.8/5  (33)
(33)
Four patterns can be noted for certain organic reactions: Pattern 1: Add a proton.
Pattern 2: Take a proton away.
Pattern 3: Reaction between an electrophile and nucleophile to form a new covalent bond.
Pattern 4: Reaction of a proton donor with a carbon-carbon double bond to form a new covalent bond.
To which pattern does the following reaction belong? 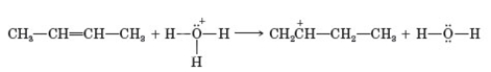
(Multiple Choice)
4.8/5  (28)
(28)
Showing 1 - 20 of 184
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)