Deck 3: Atomic Clues
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/19
Play
Full screen (f)
Deck 3: Atomic Clues
1
Which subatomic particle has the smallest mass and volume?
A) electron
B) neutron
C) nucleus
D) proton
A) electron
B) neutron
C) nucleus
D) proton
electron
2
Which scientist was responsible for the discovery of the nucleus?
A) Chadwick
B) Dalton
C) Rutherford
D) Thomson
A) Chadwick
B) Dalton
C) Rutherford
D) Thomson
Rutherford
3
Argon has three isotopes: Argon-36, Argon-38, and Argon-40. Which of these isotopes has the highest natural abundance?
A) Argon-36
B) Argon-38
C) Argon-40
A) Argon-36
B) Argon-38
C) Argon-40
Argon-40
4
Which two Greek philosophers had conflicting thoughts on the nature of the atom?
A) Galileo and Aristotle
B) Democritus and Dalton
C) Aristotle and Democritus
D) Democritus and Leucippus
A) Galileo and Aristotle
B) Democritus and Dalton
C) Aristotle and Democritus
D) Democritus and Leucippus

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
5
The abbreviated electron configuration for Cs is _____________________.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
6
Chromium has four isotopes as shown in the table.
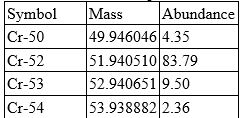 Calculate the atomic mass of chromium. Include all digits in your answer.
Calculate the atomic mass of chromium. Include all digits in your answer.
 Calculate the atomic mass of chromium. Include all digits in your answer.
Calculate the atomic mass of chromium. Include all digits in your answer.
Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate the energy emitted from a potassium atom as it releases a photon of light with a frequency of 5.99 × 1015 Hz.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
8
Explain the difference between the law of conservation of mass and the law of definite proportions.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is NOT a part of Dalton's atomic theory?
A) All matter is made up of tiny, indivisible particles called atoms.
B) Atoms cannot be created, destroyed, or transformed into other atoms in a chemical reaction.
C) All atoms of a given element contain protons, neutrons, and electrons.
D) Atoms combine in simple, whole-number ratios to form compounds.
A) All matter is made up of tiny, indivisible particles called atoms.
B) Atoms cannot be created, destroyed, or transformed into other atoms in a chemical reaction.
C) All atoms of a given element contain protons, neutrons, and electrons.
D) Atoms combine in simple, whole-number ratios to form compounds.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
10
Neon has three isotopes as shown in the table.
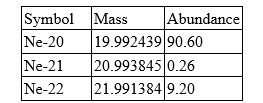 Calculate the atomic mass of neon. Include all digits in your answer.
Calculate the atomic mass of neon. Include all digits in your answer.
 Calculate the atomic mass of neon. Include all digits in your answer.
Calculate the atomic mass of neon. Include all digits in your answer.
Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
11
The full electronic configuration for Br is _____.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following configurations is the electron configuration for Cr?
A) [Ar]4s13d5
B) [Ar]3d44s2
C) [Ar]3d34s2
D) [Ar]3d6
A) [Ar]4s13d5
B) [Ar]3d44s2
C) [Ar]3d34s2
D) [Ar]3d6

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
13
Which type of spectrum is produced when light is passed through a prism?
A) continuous
B) absorption line
C) emission line
D) none of the above
A) continuous
B) absorption line
C) emission line
D) none of the above

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
14
Explain how a line spectrum is made.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
15
The full electronic configuration for Ca is _____.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
16
Lanthanum has two isotopes: La-138 and La-139. Which of these isotopes has the highest natural abundance?
A) La-138
B) La-139
A) La-138
B) La-139

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the energy emitted from a neon atom as it releases a photon of light with a wavelength of 9.582 nm.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
18
How many protons are there in In-113?
A) 49
B) 64
C) 65.8
D) 113
Enter the appropriate word(s) to complete the statement.
A) 49
B) 64
C) 65.8
D) 113
Enter the appropriate word(s) to complete the statement.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
19
The abbreviated electron configuration for xenon is _________________.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck


