Exam 3: Atomic Clues
Exam 1: Introduction to Forensic Chemistry20 Questions
Exam 2: Evidence Collection and Preservation19 Questions
Exam 3: Atomic Clues19 Questions
Exam 4: Chemical Evidence25 Questions
Exam 5: Chemistry of Bonding:20 Questions
Exam 6: Properties of Solutions I: Aqueous Solutions20 Questions
Exam 7: Properties of Solutions Ii: Intermolecular Forces and Colligative Properties24 Questions
Exam 8: Drug Chemistry25 Questions
Exam 9: Chemistry of Fire and Heat19 Questions
Exam 10: Chemistry of Explosions22 Questions
Exam 11: Applications of Chemical Kinetics20 Questions
Exam 12: Nuclear Chemistry: Energy, Medicine, Weapons, and Terrorism22 Questions
Exam 13: Chemical Equilibrium and Poisons20 Questions
Exam 14: Introduction to Biochemistry and Dna Analysis20 Questions
Select questions type
Calculate the energy emitted from a neon atom as it releases a photon of light with a wavelength of 9.582 nm.
Free
(Short Answer)
4.9/5  (38)
(38)
Correct Answer:
2.075 × 10-17 J
Which type of spectrum is produced when light is passed through a prism?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
A
Lanthanum has two isotopes: La-138 and La-139. Which of these isotopes has the highest natural abundance?
(Multiple Choice)
4.9/5  (46)
(46)
Argon has three isotopes: Argon-36, Argon-38, and Argon-40. Which of these isotopes has the highest natural abundance?
(Multiple Choice)
4.9/5  (33)
(33)
Chromium has four isotopes as shown in the table.
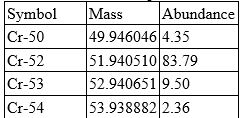 Calculate the atomic mass of chromium. Include all digits in your answer.
Calculate the atomic mass of chromium. Include all digits in your answer.
(Short Answer)
4.9/5  (33)
(33)
The abbreviated electron configuration for Cs is _____________________.
(Short Answer)
4.7/5  (29)
(29)
Calculate the energy emitted from a potassium atom as it releases a photon of light with a frequency of 5.99 × 1015 Hz.
(Short Answer)
4.8/5  (35)
(35)
The abbreviated electron configuration for xenon is _________________.
(Essay)
4.8/5  (33)
(33)
Which two Greek philosophers had conflicting thoughts on the nature of the atom?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following statements is NOT a part of Dalton's atomic theory?
(Multiple Choice)
5.0/5  (36)
(36)
Which of the following configurations is the electron configuration for Cr?
(Multiple Choice)
4.8/5  (27)
(27)
Neon has three isotopes as shown in the table.
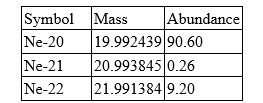 Calculate the atomic mass of neon. Include all digits in your answer.
Calculate the atomic mass of neon. Include all digits in your answer.
(Short Answer)
4.9/5  (38)
(38)
Explain the difference between the law of conservation of mass and the law of definite proportions.
(Essay)
4.8/5  (39)
(39)
Which scientist was responsible for the discovery of the nucleus?
(Multiple Choice)
4.8/5  (35)
(35)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)