Deck 11: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/72
Play
Full screen (f)
Deck 11: Acids and Bases
1
The conjugate base of 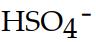 is
is
A)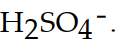
B)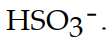
C)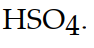
D)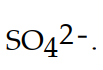
E)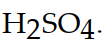
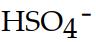 is
isA)
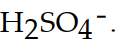
B)
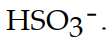
C)
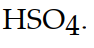
D)
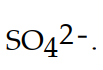
E)
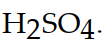
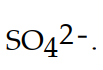
2
Which of the following is the weakest acid?
A)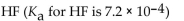
B)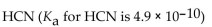
C)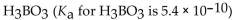
D)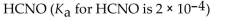
A)
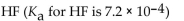
B)
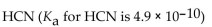
C)
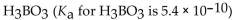
D)
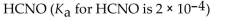
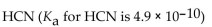
3
The name given to an aqueous solution of HNO3 is
A)hydrogen nitrate.
B)hydronitrogen acid.
C)hyponitric acid.
D)nitrous acid.
E)nitric acid.
A)hydrogen nitrate.
B)hydronitrogen acid.
C)hyponitric acid.
D)nitrous acid.
E)nitric acid.
nitric acid.
4
The conjugate acid of 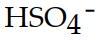 is
is
A)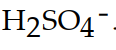
B)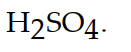
C)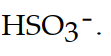
D)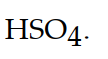
E)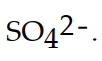
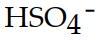 is
isA)
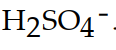
B)
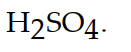
C)
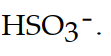
D)
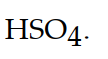
E)
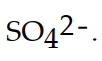

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
5
What is the 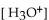 in a solution with
in a solution with 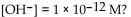
A)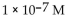
B)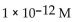
C)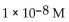
D)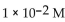
E)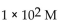
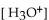 in a solution with
in a solution with 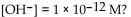
A)
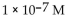
B)
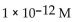
C)
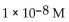
D)
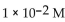
E)
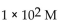

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements correctly describes the hydronium-hydroxide balance in the given solution
A)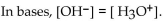
B)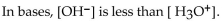
C)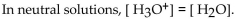
D)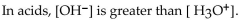
E)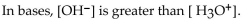
A)
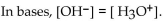
B)
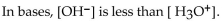
C)
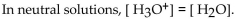
D)
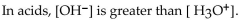
E)
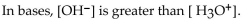

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
7
Ammonium hydroxide is a weak base because
A)it cannot hold on to its hydroxide ions.
B)it is completely ionized in aqueous solution.
C)it dissociates only slightly in water.
D)it is only slightly soluble in water.
E)it is a dilute solution.
A)it cannot hold on to its hydroxide ions.
B)it is completely ionized in aqueous solution.
C)it dissociates only slightly in water.
D)it is only slightly soluble in water.
E)it is a dilute solution.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the strongest base?
A)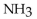
B)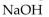
C)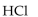
D)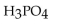
E)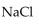
A)
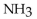
B)
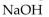
C)
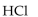
D)
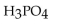
E)
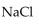

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
9
According to the Arrhenius concept, if 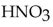 were dissolved in water, it would act as
were dissolved in water, it would act as
A) a source of hydroxide ions.
B) a base.
C) a source of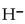 ions.
ions.
D) a proton acceptor.
E) an acid.
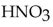 were dissolved in water, it would act as
were dissolved in water, it would act asA) a source of hydroxide ions.
B) a base.
C) a source of
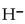 ions.
ions.D) a proton acceptor.
E) an acid.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following is characteristic of a base?
A) has a sour taste
B) has a slippery, soapy feel
C) produces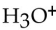 in water
in water
D) is insoluble in water
E) turns blue litmus red
A) has a sour taste
B) has a slippery, soapy feel
C) produces
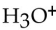 in water
in waterD) is insoluble in water
E) turns blue litmus red

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
11
According to the Brønsted-Lowry definition,
A)an acid acts as the solvent.
B)a base is a proton donor.
C)an acid is a proton acceptor.
D)a base is a proton acceptor.
E)a base produces H+ ions in aqueous solutions.
A)an acid acts as the solvent.
B)a base is a proton donor.
C)an acid is a proton acceptor.
D)a base is a proton acceptor.
E)a base produces H+ ions in aqueous solutions.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
12
According to the Arrhenius concept, if NaOH were dissolved in water, it would act as
A)a proton donor.
B)a source of hydronium ions.
C)an acid.
D)a source of H- ions.
E)a base.
A)a proton donor.
B)a source of hydronium ions.
C)an acid.
D)a source of H- ions.
E)a base.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
13
The correct formula for sulfuric acid is
A)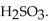
B)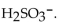
C)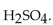
D)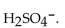
E)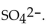
A)
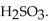
B)
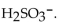
C)
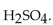
D)
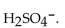
E)
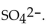

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the 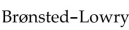 acids in the following reaction.
acids in the following reaction.
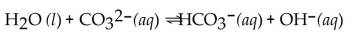
A)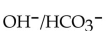
B)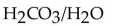
C)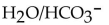
D)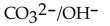
E)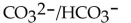
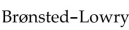 acids in the following reaction.
acids in the following reaction.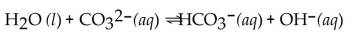
A)
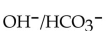
B)
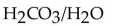
C)
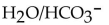
D)
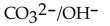
E)
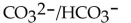

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
15
The name given to an aqueous solution of 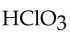 is
is
A) hypochlorous acid.
B) hydrochloric acid.
C) chlorous acid.
D) hypochloric acid.
E) chloric acid.
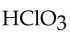 is
isA) hypochlorous acid.
B) hydrochloric acid.
C) chlorous acid.
D) hypochloric acid.
E) chloric acid.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
16
The name of 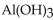 is
is
A) aluminum hydroxide.
B) aluminum trihydroxide.
C) aluminum(III) hydroxide.
D) monoaluminum trihydroxide.
E) aluminum oxygen hydride.
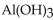 is
isA) aluminum hydroxide.
B) aluminum trihydroxide.
C) aluminum(III) hydroxide.
D) monoaluminum trihydroxide.
E) aluminum oxygen hydride.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
17
The name given to an aqueous solution of HBr is
A)hydrogen bromide.
B)bromous acid.
C)hypobromous acid.
D)hydrobromic acid.
E)bromic acid.
A)hydrogen bromide.
B)bromous acid.
C)hypobromous acid.
D)hydrobromic acid.
E)bromic acid.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
18
The conjugate base of  is
is
A)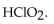
B)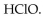
C)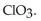
D)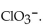
E)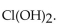
 is
isA)
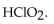
B)
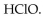
C)
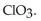
D)
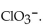
E)
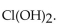

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the strongest acid?
A)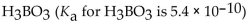
B)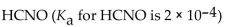
C)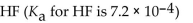
D)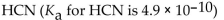
A)
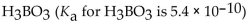
B)
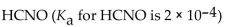
C)
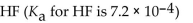
D)
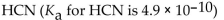

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is correctly identified?
A)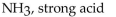
B)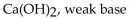
C)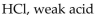
D)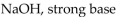
E)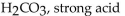
A)
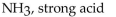
B)
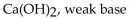
C)
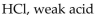
D)
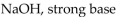
E)
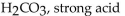

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
21
What is the pH of a solution with 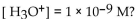
A) 9.0
B)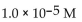
C) 5.0
D) -5.0
E) -9.0
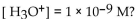
A) 9.0
B)
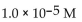
C) 5.0
D) -5.0
E) -9.0

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
22
A solution which has 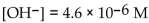 is
is
A) acidic.
B) basic.
C) neutral.
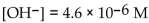 is
isA) acidic.
B) basic.
C) neutral.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
23
In which of the following are the pH values arranged from the most acidic to the most basic?
A)7, 10, 14, 4, 3, 1
B)1, 3, 6, 8, 11, 14
C)14, 10, 7, 1, 3, 5
D)14, 10, 7, 4, 3, 1
E)2, 5, 7, 9, 10, 1.1
A)7, 10, 14, 4, 3, 1
B)1, 3, 6, 8, 11, 14
C)14, 10, 7, 1, 3, 5
D)14, 10, 7, 4, 3, 1
E)2, 5, 7, 9, 10, 1.1

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
24
A solution which has 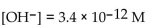 is
is
A) acidic.
B) basic.
C) neutral.
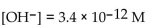 is
isA) acidic.
B) basic.
C) neutral.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
25
A patient with alkalosis has a blood pH of 7.60 . What is the 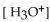 of her blood plasma?
of her blood plasma?
A)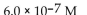
B)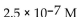
C)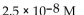
D) -7.60 M
E)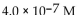
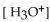 of her blood plasma?
of her blood plasma?A)
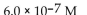
B)
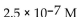
C)
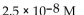
D) -7.60 M
E)
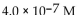

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
26
What is the pH of a solution with 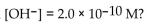
A) 9.70
B) -9.70
C) 4.30
D)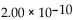
E) -4.30
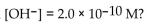
A) 9.70
B) -9.70
C) 4.30
D)
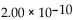
E) -4.30

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
27
What is the pH of a solution with 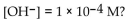
A) 4.0
B) -4.0
C)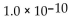
D) 10.0
E) -10.0
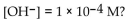
A) 4.0
B) -4.0
C)
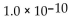
D) 10.0
E) -10.0

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
28
What is the 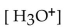 in a solution that has a
in a solution that has a 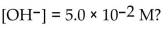
A)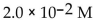
B)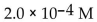
C)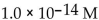
D)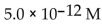
E)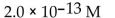
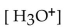 in a solution that has a
in a solution that has a 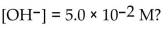
A)
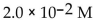
B)
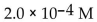
C)
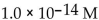
D)
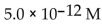
E)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
29
In which of the following are the pH values arranged from the most basic to the most acidic?
A)1, 3, 6, 8, 11, 14
B)2, 5, 7, 9, 10, 11
C)14, 10, 7, 1, 3, 5
D)14, 10, 7, 4, 3, 1
E)7, 10, 14, 4, 3, 1
A)1, 3, 6, 8, 11, 14
B)2, 5, 7, 9, 10, 11
C)14, 10, 7, 1, 3, 5
D)14, 10, 7, 4, 3, 1
E)7, 10, 14, 4, 3, 1

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
30
What is the 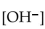 in a solution that has a
in a solution that has a 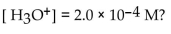
A)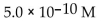
B)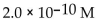
C)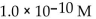
D)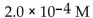
E)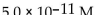
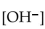 in a solution that has a
in a solution that has a 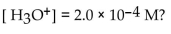
A)
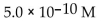
B)
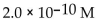
C)
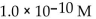
D)
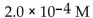
E)
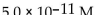

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
31
When a piece of magnesium metal is added to hydrochloric acid, what gas is produced?
A)nitrogen
B)hydrogen
C)oxygen
D)chlorine
E)carbon dioxide
A)nitrogen
B)hydrogen
C)oxygen
D)chlorine
E)carbon dioxide

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
32
What is the 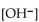 in a solution that has a
in a solution that has a 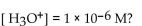
A)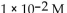
B)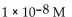
C)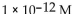
D)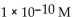
E)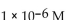
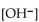 in a solution that has a
in a solution that has a 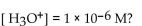
A)
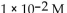
B)
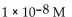
C)
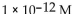
D)
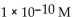
E)
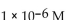

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
33
An acid and base react to form a salt and water in a(n)________ reaction.
A)dissociation
B)oxidation
C)reduction
D)ionization
E)neutralization
A)dissociation
B)oxidation
C)reduction
D)ionization
E)neutralization

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
34
What is the pH of a solution with 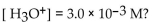
A) -2.52
B) 3.00
C) 9.00
D)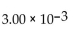
E) 2.52
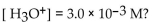
A) -2.52
B) 3.00
C) 9.00
D)
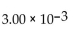
E) 2.52

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
35
The 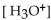 of a solution with
of a solution with 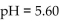 is
is
A)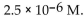
B)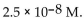
C)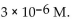
D)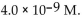
E)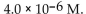
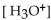 of a solution with
of a solution with 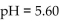 is
isA)
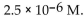
B)
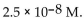
C)
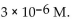
D)
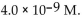
E)
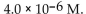

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
36
A solution which has 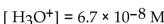 is
is
A) acidic.
B) basic.
C) neutral.
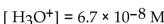 is
isA) acidic.
B) basic.
C) neutral.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
37
The 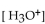 of a solution with
of a solution with 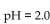 is
is
A) -10 M .
B)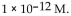
C) 10 M.
D)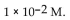
E)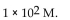
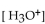 of a solution with
of a solution with 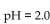 is
isA) -10 M .
B)
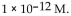
C) 10 M.
D)
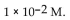
E)
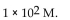

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
38
In a sulfuric acid solution, where the 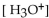 is 0.01 M, what is the pH?
is 0.01 M, what is the pH?
A) pH=11.0
B) pH=12.0
C) pH=5.0
D) pH=2.0
E) pH=3.0
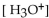 is 0.01 M, what is the pH?
is 0.01 M, what is the pH?A) pH=11.0
B) pH=12.0
C) pH=5.0
D) pH=2.0
E) pH=3.0

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
39
The 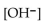 of a solution with pH=8.34 is
of a solution with pH=8.34 is
A)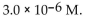
B)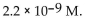
C)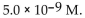
D)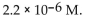
E)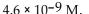
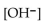 of a solution with pH=8.34 is
of a solution with pH=8.34 isA)
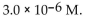
B)
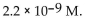
C)
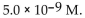
D)
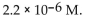
E)
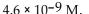

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
40
A solution with a pH of 4 is
A)extremely acidic.
B)moderately acidic.
C)neutral.
D)extremely basic.
E)slightly basic.
A)extremely acidic.
B)moderately acidic.
C)neutral.
D)extremely basic.
E)slightly basic.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
41
How many moles of 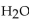 are produced when 1 mole of
are produced when 1 mole of 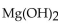 reacts with 1 mole of
reacts with 1 mole of 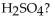
A) 1
B) 2
C) 3
D) 4
E) 5
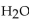 are produced when 1 mole of
are produced when 1 mole of 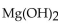 reacts with 1 mole of
reacts with 1 mole of 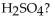
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
42
What is the molarity of a KOH solution if 25.0 mL neutralizes 35.0 mL of a 0.200 M HCl solution?
A)0.280 M
B)0.143 M
C)0.200 M
D)0.100 M
E)0.267 M
A)0.280 M
B)0.143 M
C)0.200 M
D)0.100 M
E)0.267 M

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
43
A 25.0 mL sample of 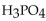 requires 50.0 mL of 1.50 M NaOH for complete neutralization. What is the molarity of the acid?
requires 50.0 mL of 1.50 M NaOH for complete neutralization. What is the molarity of the acid?
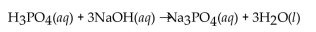
A) 0.750 M
B) 0.333 M
C) 1.50 M
D) 1.00 M
E) 3.00 M
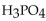 requires 50.0 mL of 1.50 M NaOH for complete neutralization. What is the molarity of the acid?
requires 50.0 mL of 1.50 M NaOH for complete neutralization. What is the molarity of the acid?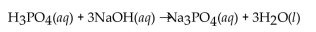
A) 0.750 M
B) 0.333 M
C) 1.50 M
D) 1.00 M
E) 3.00 M

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
44
In a buffer system of HF and its salt, NaF,
A) the HF neutralizes added acid.
B) the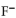 neutralizes added base.
neutralizes added base.
C) the HF neutralizes added base.
D) the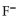 neutralizes added
neutralizes added 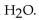
E) the HF is not necessary.
A) the HF neutralizes added acid.
B) the
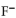 neutralizes added base.
neutralizes added base.C) the HF neutralizes added base.
D) the
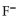 neutralizes added
neutralizes added 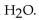
E) the HF is not necessary.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
45
In a neutralization reaction, how many moles of 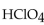 react with 1 mole of
react with 1 mole of 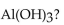
A) 1
B) 2
C) 3
D) 4
E) 5
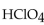 react with 1 mole of
react with 1 mole of 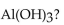
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
46
A 10.0 mL of 0.121 M 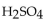 is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
A) 0.142 M
B) 0.428 M
C) 0.207 M
D) 0.0708 M
E) 0.4141 M
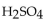 is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution isA) 0.142 M
B) 0.428 M
C) 0.207 M
D) 0.0708 M
E) 0.4141 M

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
47
The neutralization reaction between 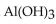 and
and 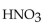 produces the salt with the formula
produces the salt with the formula
A)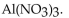
B)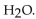
C)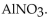
D)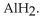
E)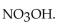
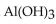 and
and 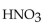 produces the salt with the formula
produces the salt with the formulaA)
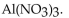
B)
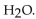
C)
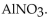
D)
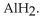
E)
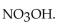

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
48


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
49
The function of a buffer is to
A)maintain the pH of a solution.
B)act as a strong acid.
C)change color at the end point of a titration.
D)maintain a neutral pH.
E)be a strong base.
A)maintain the pH of a solution.
B)act as a strong acid.
C)change color at the end point of a titration.
D)maintain a neutral pH.
E)be a strong base.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is a buffer system?
A)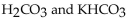
B) HCl and NaOH
C) NaCl and NaOH
D)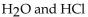
E)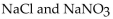
A)
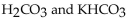
B) HCl and NaOH
C) NaCl and NaOH
D)
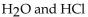
E)
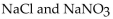

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
51
The normal blood pH is about
A)7.00.
B)7.20.
C)7.40.
D)6.80.
E)7.60.
A)7.00.
B)7.20.
C)7.40.
D)6.80.
E)7.60.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is the correctly balanced equation for the complete neutralization of 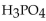 with
with 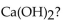
A)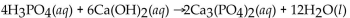
B)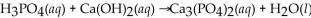
C)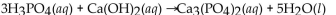
D)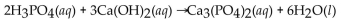
E)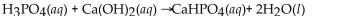
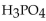 with
with 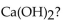
A)
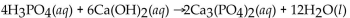
B)
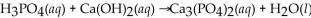
C)
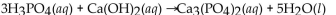
D)
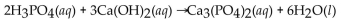
E)
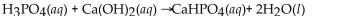

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
53
How many milliliters of 0.200 M NaOH are required to completely neutralize 5.00 mL of 0.100 M H3PO4?
A)7.50 mL
B)15.0 mL
C)0.833 mL
D)5.00 mL
E)2.50 mL
A)7.50 mL
B)15.0 mL
C)0.833 mL
D)5.00 mL
E)2.50 mL

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
54
How many milliliters of 0.100 M 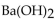 are required to neutralize 20.0 mL of 0.250 M HCl?
are required to neutralize 20.0 mL of 0.250 M HCl?
A) 25.0 mL
B) 0.250 mL
C) 100 mL
D) 0.50 mL
E) 50.0 mL
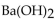 are required to neutralize 20.0 mL of 0.250 M HCl?
are required to neutralize 20.0 mL of 0.250 M HCl?A) 25.0 mL
B) 0.250 mL
C) 100 mL
D) 0.50 mL
E) 50.0 mL

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following could be a buffer?
A)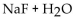
B)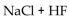
C)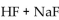
D)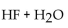
E)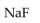
A)
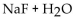
B)
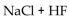
C)
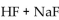
D)
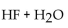
E)
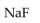

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is a neutralization reaction?
A)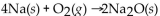
B)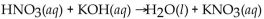
C)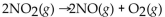
D)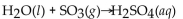
E)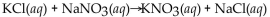
A)
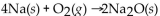
B)
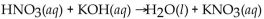
C)
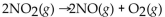
D)
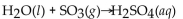
E)
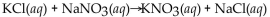

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
57
How many milliliters of 0.400 M NaOH are required to completely neutralize 20.0 mL of 0.200 M HCl?
A)10.0 mL
B)50.0 mL
C)0.100 mL
D)40.0 mL
E)20.0 mL
A)10.0 mL
B)50.0 mL
C)0.100 mL
D)40.0 mL
E)20.0 mL

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
58
25.0 mL of 0.212 M NaOH is neutralized by 13.6 mL of an HCl solution. The molarity of the HCl solution is
A)0.115 M.
B)0.212 M.
C)0.137 M.
D)0.390 M.
E)0.500 M.
A)0.115 M.
B)0.212 M.
C)0.137 M.
D)0.390 M.
E)0.500 M.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
59
In a neutralization reaction,
A)water and a salt react to form an acid and a base.
B)two acids react to form water.
C)an acid and a base react to form a salt and water.
D)an acid and a salt react to form water and a base.
E)a base and a salt react to form water and an acid.
A)water and a salt react to form an acid and a base.
B)two acids react to form water.
C)an acid and a base react to form a salt and water.
D)an acid and a salt react to form water and a base.
E)a base and a salt react to form water and an acid.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
60
A 25.0 mL sample of 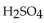 requires 20.0 mL of 2.00 M KOH for complete neutralization. What is the molarity of acid?
requires 20.0 mL of 2.00 M KOH for complete neutralization. What is the molarity of acid?
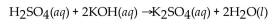
A) 0.800 M
B) 1.60 M
C) 2.00 M
D) 1.25 M
E) 2.50 M
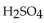 requires 20.0 mL of 2.00 M KOH for complete neutralization. What is the molarity of acid?
requires 20.0 mL of 2.00 M KOH for complete neutralization. What is the molarity of acid?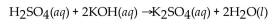
A) 0.800 M
B) 1.60 M
C) 2.00 M
D) 1.25 M
E) 2.50 M

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
61
The conjugate acid of 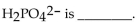
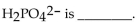

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
62
Which solution has the lowest pH?
A)a buffer made with 0.10 M acetic acid and 0.01 M sodium acetate
B)a buffer made with 0.10 M acetic acid and 0.10 M sodium acetate
C)a buffer made with 0.01 M acetic acid and 0.10 M sodium acetate
D)a buffer made with 0.01 M acetic acid and 0.01 M sodium acetate
E)All of the buffers have the same pH since they are all made with acetic acid and sodium acetate.
A)a buffer made with 0.10 M acetic acid and 0.01 M sodium acetate
B)a buffer made with 0.10 M acetic acid and 0.10 M sodium acetate
C)a buffer made with 0.01 M acetic acid and 0.10 M sodium acetate
D)a buffer made with 0.01 M acetic acid and 0.01 M sodium acetate
E)All of the buffers have the same pH since they are all made with acetic acid and sodium acetate.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
63
The name of HCl is________.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
64
When hyperventilation (rapid breathing)causes a patient to exhale large amounts of CO2, the blood pH rises in a condition called
A)pulmonary distress.
B)metabolic acidosis.
C)respiratory alkalosis.
D)respiratory acidosis.
E)metabolic alkalosis.
A)pulmonary distress.
B)metabolic acidosis.
C)respiratory alkalosis.
D)respiratory acidosis.
E)metabolic alkalosis.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
65
What is the name of the medical condition of an asthmatic patient with a blood pH of 7.30?
A)metabolic alkalosis
B)respiratory acidosis
C)respiratory alkalosis
D)diabetes mellitus
E)metabolic acidosis
A)metabolic alkalosis
B)respiratory acidosis
C)respiratory alkalosis
D)diabetes mellitus
E)metabolic acidosis

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
66
The 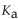 of
of 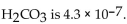
What is the pH of a buffer with 0.050 M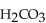 and 0.50 M
and 0.50 M 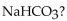
A) 7.37
B) 5.37
C) 4.3 x 10-7
D) 6.37
E) 7.63
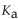 of
of 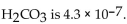
What is the pH of a buffer with 0.050 M
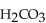 and 0.50 M
and 0.50 M 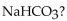
A) 7.37
B) 5.37
C) 4.3 x 10-7
D) 6.37
E) 7.63

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
67
If a condition of hyperventilation occurs, the blood pH of the patient is expected to
A)stay the same.
B)decrease.
C)concentrate.
D)saturate.
E)increase.
A)stay the same.
B)decrease.
C)concentrate.
D)saturate.
E)increase.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
68
Identify the conjugate acid-base pairs in the following reaction: 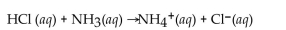
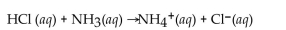

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
69
The 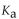 of
of 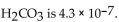
What is the pH of a buffer with 0.10 M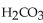 and 0.010 M
and 0.010 M 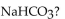
A) 6.37
B) 7.37
C) 5.37
D) 7.63
E)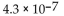
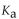 of
of 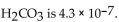
What is the pH of a buffer with 0.10 M
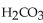 and 0.010 M
and 0.010 M 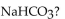
A) 6.37
B) 7.37
C) 5.37
D) 7.63
E)
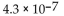

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
70
The conjugate base of 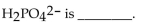

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
71
Write the proper acid dissociation expression for the ionization of acetic acid, 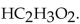
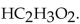

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
72
Which solution has the highest pH?
A)a buffer made with 0.10 M acetic acid and 0.01 M sodium acetate
B)a buffer made with 0.10 M acetic acid and 0.10 M sodium acetate
C)a buffer made with 0.01 M acetic acid and 0.10 M sodium acetate
D)a buffer made with 0.01 M acetic acid and 0.01 M sodium acetate
E)All of the buffers have the same pH since they are all made with acetic acid and sodium acetate.
A)a buffer made with 0.10 M acetic acid and 0.01 M sodium acetate
B)a buffer made with 0.10 M acetic acid and 0.10 M sodium acetate
C)a buffer made with 0.01 M acetic acid and 0.10 M sodium acetate
D)a buffer made with 0.01 M acetic acid and 0.01 M sodium acetate
E)All of the buffers have the same pH since they are all made with acetic acid and sodium acetate.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck


