Exam 11: Acids and Bases
Exam 1: Chemistry in Our Lives40 Questions
Exam 2: Chemistry and Measurements70 Questions
Exam 3: Matter and Energy77 Questions
Exam 4: Atoms and Elements83 Questions
Exam 5: Nuclear Chemistry64 Questions
Exam 6: Ionic and Molecular Compounds83 Questions
Exam 7: Chemical Reactions and Quantities96 Questions
Exam 8: Gases84 Questions
Exam 9: Solutions84 Questions
Exam 10: Reaction Rates and Chemical Equilibrium52 Questions
Exam 11: Acids and Bases72 Questions
Exam 12: Introduction to Organic Chemistry: Hydrocarbons105 Questions
Exam 13: Alcohols, Phenols, Thiols, and Ethers49 Questions
Exam 14: Aldehydes and Ketones44 Questions
Exam 15: Carbohydrates54 Questions
Exam 16: Carboxylic Acids and Esters55 Questions
Exam 17: Lipids70 Questions
Exam 18: Amines and Amides60 Questions
Exam 19: Amino Acids and Proteins66 Questions
Exam 20: Enzymes and Vitamins68 Questions
Exam 21: Nucleic Acids and Protein Synthesis55 Questions
Exam 22: Metabolic Pathways for Carbohydrates58 Questions
Exam 23: Metabolism and Energy Production51 Questions
Exam 24: Metabolic Pathways for Lipids and Amino Acids56 Questions
Select questions type
The correct formula for sulfuric acid is
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
What is the name of the medical condition of an asthmatic patient with a blood pH of 7.30?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following is the correctly balanced equation for the complete neutralization of 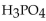 with
with 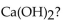
(Multiple Choice)
4.9/5  (45)
(45)
In which of the following are the pH values arranged from the most basic to the most acidic?
(Multiple Choice)
4.8/5  (41)
(41)
In a sulfuric acid solution, where the 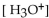 is 0.01 M, what is the pH?
is 0.01 M, what is the pH?
(Multiple Choice)
4.8/5  (45)
(45)
25.0 mL of 0.212 M NaOH is neutralized by 13.6 mL of an HCl solution. The molarity of the HCl solution is
(Multiple Choice)
4.8/5  (34)
(34)
When hyperventilation (rapid breathing)causes a patient to exhale large amounts of CO2, the blood pH rises in a condition called
(Multiple Choice)
4.9/5  (39)
(39)
When a piece of magnesium metal is added to hydrochloric acid, what gas is produced?
(Multiple Choice)
4.9/5  (44)
(44)
An acid and base react to form a salt and water in a(n)________ reaction.
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 72
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)