Deck 21: Heat and the First Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/35
Play
Full screen (f)
Deck 21: Heat and the First Law of Thermodynamics
1
A 5-g coin is dropped from a 300-m building. If it reaches a terminal velocity of 45 m/s, and the rest of the energy is converted to heating the coin, what is the change in temperature (in °C) of the coin? (The specific heat of copper is 387 J/kg⋅°C.)
A)9
B)2
C)5
D)21
E)0.5
A)9
B)2
C)5
D)21
E)0.5
5
2
Determine the heat capacity (in calories/°C) of a lake containing one million gallons (approximately 4 million kilograms) of water at 15°C.
A)4 × 106
B)4 × 109
C)4 × 103
D)1 × 103
E)4 × 102
A)4 × 106
B)4 × 109
C)4 × 103
D)1 × 103
E)4 × 102
4 × 109
3
A 5-gallon container of water (approximately 20 kg) having a temperature of 212°F is added to a 50-gallon tub (approximately 200 kg) of water having a temperature of 50°F. What is the final equilibrium temperature (in °C) of the mixture?
A)54
B)36
C)18
D)66
E)14
A)54
B)36
C)18
D)66
E)14
18
4
How much heat (in kcal) must be removed to make ice at −10°C from 2 kg of water at 20°C? (The specific heat of ice is 0.50 cal/g⋅°C.)
A)190
B)200
C)240
D)210
E)50
A)190
B)200
C)240
D)210
E)50

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
5
A 300-g glass thermometer initially at 25°C is put into 200 cm3 of hot water at 95°C. Find the final temperature (in °C) of the thermometer, assuming no heat flows to the surroundings. (The specific heat of glass is 0.2 cal/g⋅°C.)
A)52
B)68
C)89
D)79
E)36
A)52
B)68
C)89
D)79
E)36

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement below regarding the first law of thermodynamics is most correct?
A)A system can do work externally only if its internal energy decreases.
B)The internal energy of a system that interacts with its environment must change.
C)No matter what other interactions take place, the internal energy must change if a system undergoes a heat transfer.
D)The only changes that can occur in the internal energy of a system are those produced by non-mechanical forces.
E)The internal energy of a system cannot change if the heat transferred to the system is equal to the work done by the system.
A)A system can do work externally only if its internal energy decreases.
B)The internal energy of a system that interacts with its environment must change.
C)No matter what other interactions take place, the internal energy must change if a system undergoes a heat transfer.
D)The only changes that can occur in the internal energy of a system are those produced by non-mechanical forces.
E)The internal energy of a system cannot change if the heat transferred to the system is equal to the work done by the system.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
7
A child has a temperature of 101°F. If her total cross-sectional area is 2 m2, find the energy lost each second (in W) due to radiation, assuming the emissivity is 1. (Assume the room temperature is 70°F.)
A)217
B)180
C)90
D)68
E)850
A)217
B)180
C)90
D)68
E)850

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
8
How many calories of heat are required to raise the temperature of 4 kg of water from 50°F to the boiling point?
A)6.5 × 105
B)3.6 × 105
C)15 × 105
D)360
E)4 × 104
A)6.5 × 105
B)3.6 × 105
C)15 × 105
D)360
E)4 × 104

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
9
How much heat, in joules, is required to convert 1.00 kg of ice at 0°C into steam at 100°C? (LF,ice = 333 J/g; LV,steam = 2.26 × 103 J/g.)
A)3.35 × 105
B)4.19 × 105
C)2.36 × 106
D)2.69 × 106
E)3.01 × 106
A)3.35 × 105
B)4.19 × 105
C)2.36 × 106
D)2.69 × 106
E)3.01 × 106

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
10
An 8000-kg aluminum flagpole 100-m high is heated by the Sun from a temperature of 10°C to 20°C. Find the increase in internal energy (in J) of the aluminum. (The coefficient of linear expansion is 24 × 10−6 (°C)−1, the density is 2.7 × 103 kg/m3, and the specific heat of aluminum is 0.215 cal/g⋅°C.)
A)7.2 × 105
B)7.2 × 107
C)7.2 × 103
D)7.2 × 101
E)7.2 × 102
A)7.2 × 105
B)7.2 × 107
C)7.2 × 103
D)7.2 × 101
E)7.2 × 102

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
11
In an adiabatic free expansion
A)no heat is transferred between a system and its surroundings.
B)the pressure remains constant.
C)the temperature remains constant.
D)the volume remains constant.
E)the process is reversible.
A)no heat is transferred between a system and its surroundings.
B)the pressure remains constant.
C)the temperature remains constant.
D)the volume remains constant.
E)the process is reversible.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
12
A cup of coffee is enclosed on all sides in an insulated container 1/2 cm thick in the shape of a cube 10 cm on a side. The temperature of the coffee is 95°C, and the temperature of the surroundings is 21°C. Find the rate of heat loss (in J/s) due to conduction if the thermal conductivity of the cup is 2 × 10−4 cal/s⋅cm⋅°C.
A)62
B)74
C)230
D)160
E)12
A)62
B)74
C)230
D)160
E)12

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
13
One gram of water is heated from 0°C to 100°C at a constant pressure of 1 atm. Determine the approximate change in internal energy (in cal) of the water.
A)160
B)130
C)100
D)180
E)50
A)160
B)130
C)100
D)180
E)50

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
14
How much heat (in kilocalories) is needed to convert 1.00 kg of ice at 0°C into steam at 100°C?
A)23.9
B)79.6
C)564
D)643
E)720
A)23.9
B)79.6
C)564
D)643
E)720

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
15
A 5-kg piece of lead (specific heat 0.03 cal/g⋅°C) having a temperature of 80°C is added to 500 g of water having a temperature of 20°C. What is the final equilibrium temperature (in °C) of the system?
A)79
B)26
C)54
D)34
E)20
A)79
B)26
C)54
D)34
E)20

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
16
An 8000-kg aluminum flagpole 100 m long is heated by the Sun from a temperature of 10°C to 20°C. Find the work done (in J) by the aluminum if the linear expansion coefficient is 24 × 10−6 (°C)−1. (The density of aluminum is 2.7 × 103 kg/m3 and 1 atm = 1.0 × 105 N/m2.)
A)287
B)425
C)213
D)710
E)626
A)287
B)425
C)213
D)710
E)626

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
17
Five moles of an ideal gas expands isothermally at 100°C to five times its initial volume. Find the heat flow into the system.
A)2.5 × 104 J
B)1.1 × 104 J
C)6.7 × 103 J
D)2.9 × 103 J
E)7.0 × 102 J
A)2.5 × 104 J
B)1.1 × 104 J
C)6.7 × 103 J
D)2.9 × 103 J
E)7.0 × 102 J

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
18
If 25 kg of ice at 0°C is combined with 4 kg of steam at 100°C, what will be the final equilibrium temperature (in °C) of the system?
A)40
B)20
C)60
D)100
E)8
A)40
B)20
C)60
D)100
E)8

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
19
An 8000-kg aluminum flagpole 100-m long is heated by the Sun from a temperature of 10°C to 20°C. Find the heat transferred (in J) to the aluminum if the specific heat of aluminum is 0.215 cal/g⋅°C.
A)7.2 × 105
B)7.2 × 107
C)7.2 × 103
D)7.2 × 101
E)7.2 × 102
A)7.2 × 105
B)7.2 × 107
C)7.2 × 103
D)7.2 × 101
E)7.2 × 102

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
20
A slab of concrete and an insulating board are in thermal contact with each other. The temperatures of their outer surfaces are 68°F and 50°F. Determine the rate of heat transfer (in BTU/ft2⋅h) if the R values are 1.93 and 8.7 ft2⋅°F⋅h/BTU, respectively.
A)9.7
B)2.5
C)5.3
D)1.7
E)28
A)9.7
B)2.5
C)5.3
D)1.7
E)28

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
21
The theorem of equipartition of energy states that the energy each degree of freedom contributes to each molecule in the system (an ideal gas) is
A)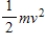
B)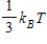
C)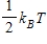
D)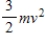
E)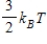
A)
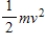
B)
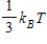
C)
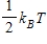
D)
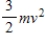
E)
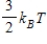

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
22
How much water at 20°C is needed to melt 1 kilogram of solid mercury at its melting point of −39°C? (The heat of fusion of mercury is 2.8 cal/gram).

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
23
In braking an automobile, the friction between the brake drums and brake shoes converts the car's kinetic energy into heat. If a 1500-kg automobile traveling at 30 m/s brakes to a halt, how much does the temperature rise in each of the four 8.0-kg brake drums? (The specific heat of each iron brake drum is 448 J/kg⋅°C).

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
24
If a person in Alaska were locked out of his house on a day when the temperature outside was −40°C, his thick clothing would mostly reduce the loss of thermal energy by
A)conduction.
B)convection.
C)radiation.
D)all of the above.
E)convection and radiation.
A)conduction.
B)convection.
C)radiation.
D)all of the above.
E)convection and radiation.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
25
Water at room temperature, 20°C, is pumped into a reactor core where it is converted to steam at 200°C. How much heat (in J) is transferred to each kilogram of water in this process? (csteam = 2 010 J/kg⋅°C; LV,steam = 2.26 × 103 J/g; 1 cal = 4.186 J.)
A)3.35 × 105
B)7.53 × 105
C)2.67 × 106
D)2.80 × 106
E)3.01 × 106
A)3.35 × 105
B)7.53 × 105
C)2.67 × 106
D)2.80 × 106
E)3.01 × 106

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
26
Beryl states that insulation with the smallest possible thermal conductivity is best to keep a house warm in winter, but worst for keeping a house cool in summer. Sapphire insists the reverse is true: low thermal conductivity is good in the summer, but bad in the winter. Which one, if either is correct?
A)Beryl, because low thermal conductivity results in low heat transfer.
B)Beryl, because low thermal conductivity results in high heat transfer.
C)Sapphire, because low thermal conductivity results in low heat transfer.
D)Sapphire, because low thermal conductivity results in high heat transfer.
E)Neither, because low heat transfer is desirable both in summer and in winter.
A)Beryl, because low thermal conductivity results in low heat transfer.
B)Beryl, because low thermal conductivity results in high heat transfer.
C)Sapphire, because low thermal conductivity results in low heat transfer.
D)Sapphire, because low thermal conductivity results in high heat transfer.
E)Neither, because low heat transfer is desirable both in summer and in winter.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
27
A styrofoam container used as a picnic cooler contains a block of ice at 0°C. If 225 grams of ice melts in 1 hour, how much heat energy per second is passing through the walls of the container? (The heat of fusion of ice is 3.33 × 105 J/kg).

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
28
A 100-g cube of ice is heated from −120°C to +120°C. In which of the following processes is the greatest amount of energy absorbed by this material?
A)warming ice to the melting point
B)melting the ice to become water
C)warming the resulting water
D)vaporizing the water to become steam
E)heating the steam
A)warming ice to the melting point
B)melting the ice to become water
C)warming the resulting water
D)vaporizing the water to become steam
E)heating the steam

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
29
In a thermodynamic process, the internal energy of a system in a container with adiabatic walls decreases by 800 J. Which statement is correct?
A)The system lost 800 J by heat transfer to its surroundings.
B)The system gained 800 J by heat transfer from its surroundings.
C)The system performed 800 J of work on its surroundings.
D)The surroundings performed 800 J of work on the system.
E)The 800 J of work done by the system was equal to the 800 J of heat transferred to the system from its surroundings.
A)The system lost 800 J by heat transfer to its surroundings.
B)The system gained 800 J by heat transfer from its surroundings.
C)The system performed 800 J of work on its surroundings.
D)The surroundings performed 800 J of work on the system.
E)The 800 J of work done by the system was equal to the 800 J of heat transferred to the system from its surroundings.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following substances has the greatest specific heat?
A)copper
B)ice
C)water
D)steam
E)Ice, water, and steam have equal specific heats since they are the same material, and this specific heat is greater than that of copper.
A)copper
B)ice
C)water
D)steam
E)Ice, water, and steam have equal specific heats since they are the same material, and this specific heat is greater than that of copper.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
31
Duff states that equal masses of all substances have equal changes in internal energy when they have equal changes in temperature. Javan states that the change in internal energy is equal to a constant times the change in temperature for every ΔT, no matter how large or how small ΔT is, but that the constant is different for different substances. Which one, if either, is correct?
A)Neither, because the specific heat depends on the substance and may vary with temperature.
B)Neither, because a change of state may involve release or absorption of latent heat.
C)Neither because a substance may do work during a temperature change.
D)All of the statements above are correct.
E)Only statements (a) and (b) are correct.
A)Neither, because the specific heat depends on the substance and may vary with temperature.
B)Neither, because a change of state may involve release or absorption of latent heat.
C)Neither because a substance may do work during a temperature change.
D)All of the statements above are correct.
E)Only statements (a) and (b) are correct.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
32
A gas expands as shown in the graph. If the heat taken in during this process is 1.02 × 106 J and 1 atm = 1.01 × 105 N/m2, the change in internal energy of the gas (in J) is how much? 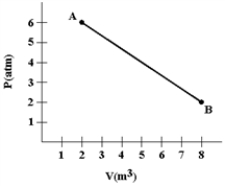
A)−2.42 × 106
B)−1.40 × 106
C)−1.02 × 106
D)1.02 × 106
E)1.40 × 106

A)−2.42 × 106
B)−1.40 × 106
C)−1.02 × 106
D)1.02 × 106
E)1.40 × 106

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
33
We are able to define a mechanical equivalent for heat because
A)some thermal energy can be converted into mechanical energy.
B)mechanical energy can be converted into thermal energy.
C)work can be converted into thermal energy.
D)some thermal energy can be converted into work.
E)all of the above can occur.
A)some thermal energy can be converted into mechanical energy.
B)mechanical energy can be converted into thermal energy.
C)work can be converted into thermal energy.
D)some thermal energy can be converted into work.
E)all of the above can occur.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
34
A block of material of mass m and specific heat c falls from height h and reaches speed v just before striking the ground. Its temperature is measured immediately after it strikes the ground. If we ignore any change in temperature owing to interaction with the air, the change in temperature of the block of material is
A)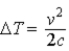 .
.
B)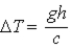 .
.
C)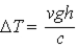 .
.
D)All of the answers above are correct.
E)Only (a) and (b) above are correct.
A)
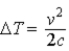 .
.B)
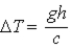 .
.C)
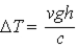 .
.D)All of the answers above are correct.
E)Only (a) and (b) above are correct.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
35
100 grams of liquid nitrogen at 77 K is stirred into a beaker containing 200 grams of 5°C water. If the nitrogen leaves the solution as soon as it turns to gas, how much water freezes? (The heat of evaporation of nitrogen is 6.09 cal/gram and the heat of fusion of water is 80 cal/gram.)

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck


