Exam 21: Heat and the First Law of Thermodynamics
Exam 1: Getting Started24 Questions
Exam 2: One-Dimensional Motion66 Questions
Exam 3: Vectors47 Questions
Exam 4: Two- and Three-Dimensional Motion79 Questions
Exam 5: Newtons Laws of Motion103 Questions
Exam 6: Applications of Newtons Laws of Motion64 Questions
Exam 7: Gravity47 Questions
Exam 8: Conservation of Energy31 Questions
Exam 9: Energy in Nonisolated Systems41 Questions
Exam 10: Systems of Particles and Conservation of Momentum25 Questions
Exam 11: Collisions43 Questions
Exam 12: Rotation I: Kinematics and Dynamics65 Questions
Exam 13: Rotation II: a Conservation Approach42 Questions
Exam 14: Static Equilibrium, Elasticity, and Fracture34 Questions
Exam 15: Fluids53 Questions
Exam 16: Oscillations41 Questions
Exam 17: Traveling Waves46 Questions
Exam 18: Superposition and Standing Waves56 Questions
Exam 19: Temperature, Thermal Expansion, and Gas Laws45 Questions
Exam 20: Kinetic Theory of Gases19 Questions
Exam 21: Heat and the First Law of Thermodynamics35 Questions
Exam 22: Entropy and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Forces34 Questions
Exam 24: Electric Fields48 Questions
Exam 25: Gausss Law80 Questions
Exam 26: Electric Potential96 Questions
Exam 27: Capacitors and Batteries63 Questions
Exam 28: Current and Resistance32 Questions
Exam 29: Direct Current Dc Circuits84 Questions
Exam 30: Magnetic Fields and Forces75 Questions
Exam 31: Gausss Law for Magnetism and Amperes Law87 Questions
Exam 32: Faradays Law of Induction56 Questions
Exam 33: Inductors and Ac Circuits86 Questions
Exam 34: Maxwells Equations and Electromagnetic Waves41 Questions
Exam 35: Diffraction and Interference48 Questions
Exam 36: Applications of the Wave Model31 Questions
Exam 37: Reflection and Images Formed by Reflection25 Questions
Exam 38: Refraction and Images Formed by Refraction54 Questions
Exam 39: Relativity45 Questions
Select questions type
Duff states that equal masses of all substances have equal changes in internal energy when they have equal changes in temperature. Javan states that the change in internal energy is equal to a constant times the change in temperature for every ΔT, no matter how large or how small ΔT is, but that the constant is different for different substances. Which one, if either, is correct?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
A gas expands as shown in the graph. If the heat taken in during this process is 1.02 × 106 J and 1 atm = 1.01 × 105 N/m2, the change in internal energy of the gas (in J) is how much? 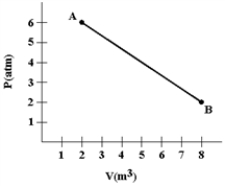
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
B
A 100-g cube of ice is heated from −120°C to +120°C. In which of the following processes is the greatest amount of energy absorbed by this material?
Free
(Multiple Choice)
4.8/5  (46)
(46)
Correct Answer:
D
How much water at 20°C is needed to melt 1 kilogram of solid mercury at its melting point of −39°C? (The heat of fusion of mercury is 2.8 cal/gram).
(Short Answer)
4.8/5  (33)
(33)
A 300-g glass thermometer initially at 25°C is put into 200 cm3 of hot water at 95°C. Find the final temperature (in °C) of the thermometer, assuming no heat flows to the surroundings. (The specific heat of glass is 0.2 cal/g⋅°C.)
(Multiple Choice)
4.9/5  (31)
(31)
A styrofoam container used as a picnic cooler contains a block of ice at 0°C. If 225 grams of ice melts in 1 hour, how much heat energy per second is passing through the walls of the container? (The heat of fusion of ice is 3.33 × 105 J/kg).
(Short Answer)
4.8/5  (37)
(37)
The theorem of equipartition of energy states that the energy each degree of freedom contributes to each molecule in the system (an ideal gas) is
(Multiple Choice)
4.9/5  (38)
(38)
An 8000-kg aluminum flagpole 100-m high is heated by the Sun from a temperature of 10°C to 20°C. Find the increase in internal energy (in J) of the aluminum. (The coefficient of linear expansion is 24 × 10−6 (°C)−1, the density is 2.7 × 103 kg/m3, and the specific heat of aluminum is 0.215 cal/g⋅°C.)
(Multiple Choice)
4.8/5  (21)
(21)
How much heat (in kcal) must be removed to make ice at −10°C from 2 kg of water at 20°C? (The specific heat of ice is 0.50 cal/g⋅°C.)
(Multiple Choice)
4.8/5  (32)
(32)
Which statement below regarding the first law of thermodynamics is most correct?
(Multiple Choice)
4.7/5  (36)
(36)
If a person in Alaska were locked out of his house on a day when the temperature outside was −40°C, his thick clothing would mostly reduce the loss of thermal energy by
(Multiple Choice)
4.8/5  (43)
(43)
In a thermodynamic process, the internal energy of a system in a container with adiabatic walls decreases by 800 J. Which statement is correct?
(Multiple Choice)
4.9/5  (28)
(28)
Determine the heat capacity (in calories/°C) of a lake containing one million gallons (approximately 4 million kilograms) of water at 15°C.
(Multiple Choice)
4.7/5  (45)
(45)
A 5-gallon container of water (approximately 20 kg) having a temperature of 212°F is added to a 50-gallon tub (approximately 200 kg) of water having a temperature of 50°F. What is the final equilibrium temperature (in °C) of the mixture?
(Multiple Choice)
4.8/5  (34)
(34)
In braking an automobile, the friction between the brake drums and brake shoes converts the car's kinetic energy into heat. If a 1500-kg automobile traveling at 30 m/s brakes to a halt, how much does the temperature rise in each of the four 8.0-kg brake drums? (The specific heat of each iron brake drum is 448 J/kg⋅°C).
(Short Answer)
4.9/5  (41)
(41)
Which of the following substances has the greatest specific heat?
(Multiple Choice)
5.0/5  (32)
(32)
An 8000-kg aluminum flagpole 100-m long is heated by the Sun from a temperature of 10°C to 20°C. Find the heat transferred (in J) to the aluminum if the specific heat of aluminum is 0.215 cal/g⋅°C.
(Multiple Choice)
4.9/5  (33)
(33)
We are able to define a mechanical equivalent for heat because
(Multiple Choice)
4.9/5  (40)
(40)
A slab of concrete and an insulating board are in thermal contact with each other. The temperatures of their outer surfaces are 68°F and 50°F. Determine the rate of heat transfer (in BTU/ft2⋅h) if the R values are 1.93 and 8.7 ft2⋅°F⋅h/BTU, respectively.
(Multiple Choice)
4.9/5  (36)
(36)
A 5-g coin is dropped from a 300-m building. If it reaches a terminal velocity of 45 m/s, and the rest of the energy is converted to heating the coin, what is the change in temperature (in °C) of the coin? (The specific heat of copper is 387 J/kg⋅°C.)
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 35
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)