Deck 20: Hydrogen
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 20: Hydrogen
1
(a) Given a fuel cell efficiency of 75% and a required energy to the wheels 

(a) The input energy for the fuel cell is (0.6 MJ/km)/(0.75) = 0.8 MJ/km. Since 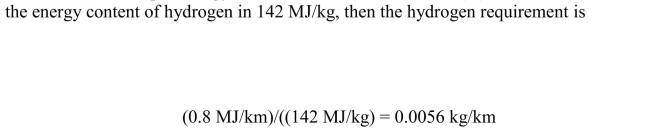

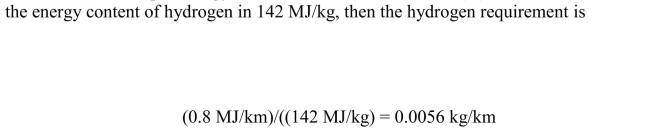

2
Consider the possibility of extracting energy from 1 m3 of water. One
approach would be lift the water to some elevation and then to generate electricity
hydroelectrically (i.e., pumped hydroelectric storage). The second approach would be to
produce hydrogen from the water by electrolysis and then generate electricity from the
hydrogen in a proton exchange membrane fuel cell. Using typical efficiencies as given in
Chapters 18 and 20, how high would the cubic meter of water have to be lifted to provide
the same total electrical energy output as the fuel cell?
approach would be lift the water to some elevation and then to generate electricity
hydroelectrically (i.e., pumped hydroelectric storage). The second approach would be to
produce hydrogen from the water by electrolysis and then generate electricity from the
hydrogen in a proton exchange membrane fuel cell. Using typical efficiencies as given in
Chapters 18 and 20, how high would the cubic meter of water have to be lifted to provide
the same total electrical energy output as the fuel cell?
The gravitational potential energy is 

3
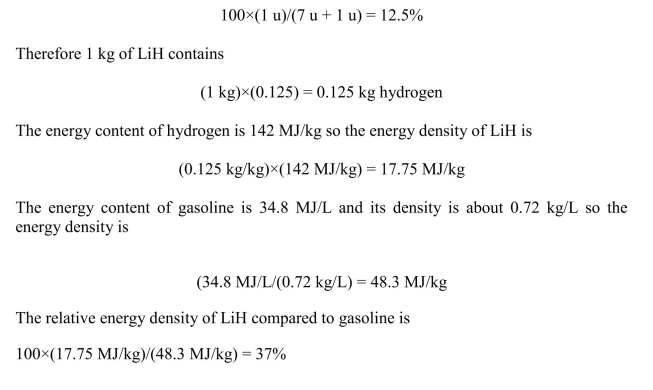
The lightest element that is a solid at room temperature, lithium, absorbs 

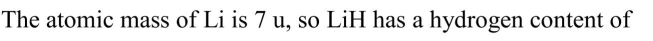
4
Consider the following three applications of fuel cells for producing 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Hydrogen gas is burned to provide heat for a typical North American home
(see Chapter 2 for heating requirements). If the hydrogen is stored in a spherical tank at a
pressure of 80 MPa, what would be the diameter of the tank needed to supply the average
monthly heating requirement? Assume a typical furnace efficiency (Chapter 17).
(see Chapter 2 for heating requirements). If the hydrogen is stored in a spherical tank at a
pressure of 80 MPa, what would be the diameter of the tank needed to supply the average
monthly heating requirement? Assume a typical furnace efficiency (Chapter 17).

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
A solid oxide fuel cell operates at 55% efficiency using methane as a fuel. 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
One possible use for hydrogen as an energy storage mechanism is for 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Hydrogen gas is burned according to the reaction 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Assume that the cost of hydrogen per unit energy is the same as gasoline 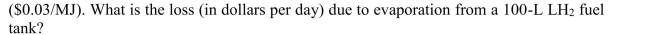
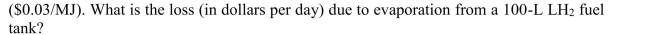

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the situation where all 250,000,000 gasoline-powered vehicles in 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
For stationary applications, mass and volume considerations for a fuel 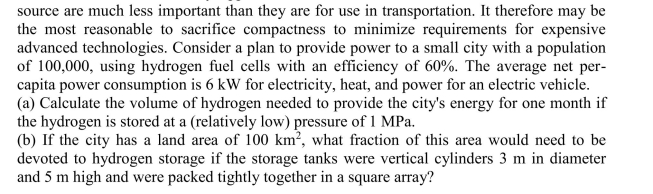


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
If hydrogen followed an ideal gas law at all pressures, calculate the pressure 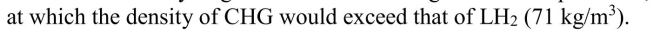
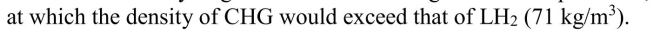

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
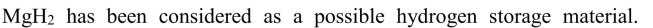 Calculate the mass and volume of Mg that would be required to store 100 kg of hydrogen.
Calculate the mass and volume of Mg that would be required to store 100 kg of hydrogen.
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
The BMW Hydrogen 7 consumes an average of 13.7 L/100 km of gasoline 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
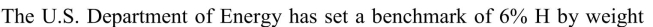
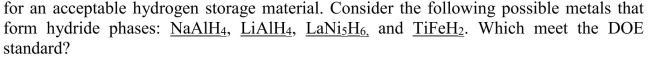

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate the percentage of improvement in the energy density of a CHG 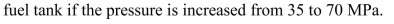
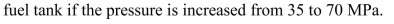

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the carbon footprint /km) for a fuel cell vehicle 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
The Mazda RX-8 RE hydrogen vehicle has a 110 L CHG tank that stores 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
If the BMW Hydrogen 7 consumes an average of 13.7 L/100 km when 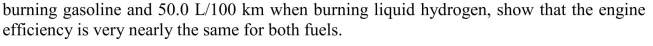
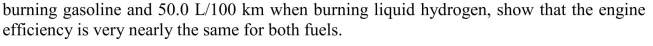

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Consider a simple model of the burning of butane as the oxidation of the 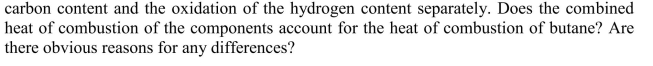
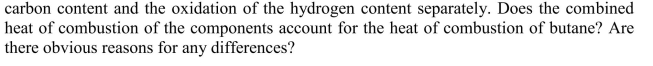

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


