Deck 7: Energy and Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 7: Energy and Metabolism
1
Consider the following two chemical equations: A. glucose + fructose → sucrose + H2O, Δ G = +27 kJ/mole (or +6.5 kcal/mole)
B) glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = −5 kJ/mole (or −1.2 kcal/mole)
What is the overall Δ G ?
A) 22 kJ/mole
B) 32 kJ/mole
C) -32 kJ/mole
D) −135 kJ/mole
E) 135 kJ/mole
B) glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = −5 kJ/mole (or −1.2 kcal/mole)
What is the overall Δ G ?
A) 22 kJ/mole
B) 32 kJ/mole
C) -32 kJ/mole
D) −135 kJ/mole
E) 135 kJ/mole
A
2
In a reaction in which the rate of the reverse reaction is equal to the rate of the forward reaction, a state of ____ is attained.
A) total entropy
B) enthalpy
C) thermodynamics
D) dynamic equilibrium
E) product reversibility
A) total entropy
B) enthalpy
C) thermodynamics
D) dynamic equilibrium
E) product reversibility
D
3
Every type of chemical bond contains a certain amount of energy. The total bond energy, which is essentially equivalent to the total potential energy of the system, is a quantity known as
A) entropy.
B) enthalpy.
C) free energy.
D) kinetic energy.
E) thermodynamic energy.
A) entropy.
B) enthalpy.
C) free energy.
D) kinetic energy.
E) thermodynamic energy.
B
4
The unit of measurement for energy that most biologists today prefer is ____________.
A) kilowatts
B) kilograms
C) kilocalories
D) kilometers
E) kilojoules
A) kilowatts
B) kilograms
C) kilocalories
D) kilometers
E) kilojoules

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
What is the ultimate source of energy for almost all living organisms?
A) Heat
B) Lipids
C) The sun
D) Glucose
E) Carbon dioxide
A) Heat
B) Lipids
C) The sun
D) Glucose
E) Carbon dioxide

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
Why type of energy is represented by a positive change in G ?
A) Entropy
B) Enthalpy
C) Exergonic
D) Endergonic
E) Activation
A) Entropy
B) Enthalpy
C) Exergonic
D) Endergonic
E) Activation

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
An organism can exchange matter and energy with its surroundings. Thus, any change in an organism's energy content must be balanced by a corresponding change in the energy content of the surroundings. As such, an organism is referred to as a(n)
A) closed system.
B) open system.
C) dynamic system.
D) potential system.
E) thermally reactive system.
A) closed system.
B) open system.
C) dynamic system.
D) potential system.
E) thermally reactive system.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
In order for a cell to maintain a high degree of order, it must constantly
A) use energy.
B) produce energy.
C) destroy energy.
D) release energy.
E) increase energy.
A) use energy.
B) produce energy.
C) destroy energy.
D) release energy.
E) increase energy.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
Capacity to do work as a result of position or state is known as _______.
A) potential energy
B) thermal energy
C) electrical energy
D) chemical energy
E) kinetic energy
A) potential energy
B) thermal energy
C) electrical energy
D) chemical energy
E) kinetic energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
An endergonic reaction can proceed only if it absorbs
A) randomly available free energy.
B) less free energy than is released by a coupled exergonic reaction.
C) more free energy than is released by a coupled exergonic reaction.
D) less free energy than is released by a coupled endergonic reaction.
E) the same amount of free energy that is absorbed by the enzymatic breakdown of proteins.
A) randomly available free energy.
B) less free energy than is released by a coupled exergonic reaction.
C) more free energy than is released by a coupled exergonic reaction.
D) less free energy than is released by a coupled endergonic reaction.
E) the same amount of free energy that is absorbed by the enzymatic breakdown of proteins.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
Suppose the free energy of the reactants is greater than the free energy of the products. Such a reaction is referred to as a(n) ____.
A) entropic reaction
B) endergonic reaction
C) exergonic reaction
D) catabolic reaction
E) activation reaction
A) entropic reaction
B) endergonic reaction
C) exergonic reaction
D) catabolic reaction
E) activation reaction

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
According to the second law of thermodynamics, the amount of usable energy available to do work in the universe ____ over time.
A) crashes
B) decreases
C) increases
D) stays constant
E) exponentially increases
A) crashes
B) decreases
C) increases
D) stays constant
E) exponentially increases

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
What unit is used to measure thermal energy that flows from an object of higher temperature to an object of lower temperature?
A) Kilograms
B) Kilometers
C) Kilowatts
D) Kilocalories
E) Kilojoules
A) Kilograms
B) Kilometers
C) Kilowatts
D) Kilocalories
E) Kilojoules

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
As you climb a flight of stairs, what type of energy are your legs in motion using?
A) Kinetic energy
B) Thermal energy
C) Chemical energy
D) Electrical energy
E) Potential energy
A) Kinetic energy
B) Thermal energy
C) Chemical energy
D) Electrical energy
E) Potential energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
You are out cycling and start to climb a hill. As you crest the hill, just before cycling down, what kind of energy have you stored?
A) Kinetic energy
B) Thermal energy
C) Chemical energy
D) Electrical energy
E) Potential energy
A) Kinetic energy
B) Thermal energy
C) Chemical energy
D) Electrical energy
E) Potential energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
Which best describes a catabolic reaction?
A) It involves the expenditure of energy.
B) It involves the anabolic production of complex molecules.
C) It involves the breakdown of life sustaining processes within cells.
D) It involves the breakdown of large organic molecules to simple building blocks.
E) It involves the synthesis of complex organic molecules from simple building blocks.
A) It involves the expenditure of energy.
B) It involves the anabolic production of complex molecules.
C) It involves the breakdown of life sustaining processes within cells.
D) It involves the breakdown of large organic molecules to simple building blocks.
E) It involves the synthesis of complex organic molecules from simple building blocks.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is contrary to the second law of thermodynamics?
A) Total entropy in the universe is decreasing with time.
B) When energy is used, it is irreplaceable.
C) Energy is converted from one form to another form.
D) Energy can be transferred from one form to another.
E) Total amount of energy in the universe that is available to do work decreases over time.
A) Total entropy in the universe is decreasing with time.
B) When energy is used, it is irreplaceable.
C) Energy is converted from one form to another form.
D) Energy can be transferred from one form to another.
E) Total amount of energy in the universe that is available to do work decreases over time.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
Total bond energy is essentially equivalent to the total potential energy of the system, a quantity known as ____________.
A) exergonic
B) enthalpy
C) activation
D) entropy
E) endergonic
A) exergonic
B) enthalpy
C) activation
D) entropy
E) endergonic

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
Energy that is usable and organized is classified as having ____.
A) low energy
B) low entropy
C) low enthalpy
D) high entropy
E) high energy
A) low energy
B) low entropy
C) low enthalpy
D) high entropy
E) high energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is contrary to the first law of thermodynamics?
A) When energy is used, it is irreplaceable.
B) Matter can be converted into energy.
C) The amount of energy in the universe is constant.
D) Energy can be converted from one form to another.
E) Energy can be transferred from one form to another.
A) When energy is used, it is irreplaceable.
B) Matter can be converted into energy.
C) The amount of energy in the universe is constant.
D) Energy can be converted from one form to another.
E) Energy can be transferred from one form to another.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following two chemical equations: A. glucose + fructose → sucrose + H2O, Δ G = +27 kJ/mole (or +6.5 kcal/mole)
B) glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = −5 kJ/mole (or −1.2 kcal/mole)
The overall free energy change in the chemical equations (
A) and (B) above is accomplished by
A) a decrease in activation energy.
B) combining two endergonic reactions.
C) combining an endergonic and an exergonic reaction.
D) combining two exergonic reactions.
E) measuring the reaction rate.
B) glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = −5 kJ/mole (or −1.2 kcal/mole)
The overall free energy change in the chemical equations (
A) and (B) above is accomplished by
A) a decrease in activation energy.
B) combining two endergonic reactions.
C) combining an endergonic and an exergonic reaction.
D) combining two exergonic reactions.
E) measuring the reaction rate.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
What form of energy is stored within the molecules of ATP?
A) Kinetic
B) Heat
C) Potential
D) Nuclear
E) Light
A) Kinetic
B) Heat
C) Potential
D) Nuclear
E) Light

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
The region of the enzyme molecule that interacts with a substrate is called the ______.
A) cofactor
B) active site
C) reaction site
D) enzyme product site
E) enzyme-substrate complex
A) cofactor
B) active site
C) reaction site
D) enzyme product site
E) enzyme-substrate complex

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
Which best describes the energy of activation?
A) A type of exergonic reaction
B) A type of endergonic reaction
C) The energy required to break existing bonds
D) The catalysts needed to raise a reaction's rate
E) The enzymes required to lower a reaction's rate
A) A type of exergonic reaction
B) A type of endergonic reaction
C) The energy required to break existing bonds
D) The catalysts needed to raise a reaction's rate
E) The enzymes required to lower a reaction's rate

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
What refers to the alteration of an enzyme's shape due to binding of substrate?
A) Active site
B) Cofactor
C) Induced fit
D) Activation energy
E) Allosteric inhibition
A) Active site
B) Cofactor
C) Induced fit
D) Activation energy
E) Allosteric inhibition

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
Which class of enzyme can catalyze the conversion of a molecule from one isomeric form to another?
A) Hydrolases
B) Lyases
C) Ligases
D) Isomerases
E) Transferases
A) Hydrolases
B) Lyases
C) Ligases
D) Isomerases
E) Transferases

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
Figure 7-2
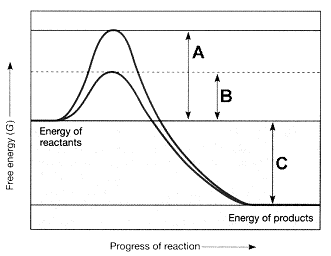
Refer to the accompanying figure. The line on the graph labeled B represents the ____.
A) change in entropy
B) change in enthalpy
C) free energy of the reactants
D) activation energy with an enzyme
E) activation energy without an enzyme

Refer to the accompanying figure. The line on the graph labeled B represents the ____.
A) change in entropy
B) change in enthalpy
C) free energy of the reactants
D) activation energy with an enzyme
E) activation energy without an enzyme

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is a hydrogen ion acceptor?
A) ATP
B) ADP
C) FAD
D) FADH2
E) NADPH
A) ATP
B) ADP
C) FAD
D) FADH2
E) NADPH

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
The direction of an exergonic reaction can best be described as moving
A) from higher to lower entropy.
B) from lower to higher free energy.
C) from higher to lower free energy.
D) from higher to lower absolute temperature.
E) from lower to higher absolute temperature.
A) from higher to lower entropy.
B) from lower to higher free energy.
C) from higher to lower free energy.
D) from higher to lower absolute temperature.
E) from lower to higher absolute temperature.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
Which chemical process occurs in which a substance loses electrons?
A) Entropy
B) Enthalpy
C) Oxidation
D) Reduction
E) Anabolism
A) Entropy
B) Enthalpy
C) Oxidation
D) Reduction
E) Anabolism

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statement is correct in the context of this equation H = G + TS?
A) Both enthalpy and free energy remain the same.
B) As the temperature increases, there is a decrease in random molecular motion.
C) As entropy decreases, the amount of free energy decreases.
D) As entropy increases, the amount of free energy decreases.
E) As entropy increases, the amount of free energy increases.
A) Both enthalpy and free energy remain the same.
B) As the temperature increases, there is a decrease in random molecular motion.
C) As entropy decreases, the amount of free energy decreases.
D) As entropy increases, the amount of free energy decreases.
E) As entropy increases, the amount of free energy increases.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is the common cofactor for enzymes?
A) Zinc
B) Copper
C) Lead
D) Iron
E) Magnesium
A) Zinc
B) Copper
C) Lead
D) Iron
E) Magnesium

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
The equation, G = H − TS , predicts that
A) metabolism decreases proportionately to anabolism.
B) metabolism increases proportionately to catabolism.
C) as enthalpy decreases, the amount of entropy also decreases.
D) as entropy increases, the amount of free energy decreases.
E) as enthalpy increases, the amount of free energy increases.
A) metabolism decreases proportionately to anabolism.
B) metabolism increases proportionately to catabolism.
C) as enthalpy decreases, the amount of entropy also decreases.
D) as entropy increases, the amount of free energy decreases.
E) as enthalpy increases, the amount of free energy increases.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
What is needed in the following reaction: ____ + Pi + energy → ADP.
A) ATP
B) H2O
C) AMP
D) cAMP
E) glucose-P
A) ATP
B) H2O
C) AMP
D) cAMP
E) glucose-P

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
The reaction ATP + H2O → ADP + Pi is classified as an
A) endergonic reaction.
B) enthalpy reaction.
C) entropy reaction.
D) exergonic reaction.
E) electrochemical reaction.
A) endergonic reaction.
B) enthalpy reaction.
C) entropy reaction.
D) exergonic reaction.
E) electrochemical reaction.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
The maintenance of a high ATP to ADP ratio within cells ensures that
A) the hydrolysis of ADP to ATP will be strongly exergonic.
B) the hydrolysis of ATP to ADP will be strongly exergonic.
C) the hydrolysis of ATP to ADP will be strongly endergonic.
D) the hydrolysis of ADP to ATP will be an energy releasing reaction.
E) the conversion of ADP to ATP will proceed spontaneously.
A) the hydrolysis of ADP to ATP will be strongly exergonic.
B) the hydrolysis of ATP to ADP will be strongly exergonic.
C) the hydrolysis of ATP to ADP will be strongly endergonic.
D) the hydrolysis of ADP to ATP will be an energy releasing reaction.
E) the conversion of ADP to ATP will proceed spontaneously.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
Why are enzymes considered important biological catalysts?
A) Enzymes lower the entropy of a biochemical reaction.
B) Enzymes decrease the enthalpy of a biochemical reaction.
C) Enzymes increase the free energy of a biochemical reaction.
D) Enzymes supply the energy to initiate a biochemical reaction.
E) Enzymes lower the activation energy of a biochemical reaction.
A) Enzymes lower the entropy of a biochemical reaction.
B) Enzymes decrease the enthalpy of a biochemical reaction.
C) Enzymes increase the free energy of a biochemical reaction.
D) Enzymes supply the energy to initiate a biochemical reaction.
E) Enzymes lower the activation energy of a biochemical reaction.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
Select the phosphorylation reaction.
A) Glucose + fructose → sucrose + H2O
B) Glucose + ATP → glucose-P + ADP
C) Glucose-P + fructose → sucrose + Pi
D) Glucose + glucose → maltose
E) Sucrose + H2O → glucose + fructose
A) Glucose + fructose → sucrose + H2O
B) Glucose + ATP → glucose-P + ADP
C) Glucose-P + fructose → sucrose + Pi
D) Glucose + glucose → maltose
E) Sucrose + H2O → glucose + fructose

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
Figure 7-2
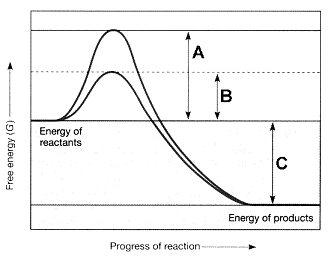
Refer to the accompanying figure. The line on the graph labeled C represents the ____.
A) change in entropy
B) change in enthalpy
C) change in free energy
D) activation energy with an enzyme
E) activation energy without an enzyme

Refer to the accompanying figure. The line on the graph labeled C represents the ____.
A) change in entropy
B) change in enthalpy
C) change in free energy
D) activation energy with an enzyme
E) activation energy without an enzyme

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
Select the hydrogen acceptor molecule that stores electrons in the process of photosynthesis.
A) Nicotinamide adenine dinucleotide phosphate (NADP+)
B) Nicotinamide adenine dinucleotide (NAD+)
C) Flavin adenine dinucleotide (FAD)
D) Adenine triphosphate (ATP)
E) Adenine diphosphate (ADP)
A) Nicotinamide adenine dinucleotide phosphate (NADP+)
B) Nicotinamide adenine dinucleotide (NAD+)
C) Flavin adenine dinucleotide (FAD)
D) Adenine triphosphate (ATP)
E) Adenine diphosphate (ADP)

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
A nonpolypeptide compound that binds to the apoenzyme and serves as a cofactor is called
A) starch.
B) pyruvic acid.
C) serine.
D) heme.
E) coenzyme.
A) starch.
B) pyruvic acid.
C) serine.
D) heme.
E) coenzyme.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
Which refers to an organic, non-polypeptide compound that binds to the apoenzyme and serves as a cofactor?
A) Mineral
B) Catalyst
C) Substrate
D) Coenzyme
E) Allosteric regulator
A) Mineral
B) Catalyst
C) Substrate
D) Coenzyme
E) Allosteric regulator

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
Many bacterial infections are treated with drugs, such as penicillin and sulpha drugs, that ____ the bacteria's enzyme activity.
A) inhibit
B) promote
C) enhance
D) stabilize
E) accentuate
A) inhibit
B) promote
C) enhance
D) stabilize
E) accentuate

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
_____________ is a protein-digesting enzyme in the very acidic stomach juice.
A) Trypsin
B) Chymotrypsin
C) Elastase
D) Pepsin
E) Dipeptidase
A) Trypsin
B) Chymotrypsin
C) Elastase
D) Pepsin
E) Dipeptidase

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
What is the function of the oxidoreductases?
A) Catalyze conversion of a molecule from one isomeric form to another
B) Catalyze hydrolysis reactions
C) Catalyze oxidation-reduction reactions
D) Catalyze certain reactions in which double bonds form or break
E) Catalyze the transfer of a functional group from a donor molecule to an acceptor molecule
A) Catalyze conversion of a molecule from one isomeric form to another
B) Catalyze hydrolysis reactions
C) Catalyze oxidation-reduction reactions
D) Catalyze certain reactions in which double bonds form or break
E) Catalyze the transfer of a functional group from a donor molecule to an acceptor molecule

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
The conversion of polysaccharides to monosaccharides is an example of an anabolic reaction.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
How do competitive inhibitors hinder biochemical reactions?
A) Competitive inhibitors denature the enzyme.
B) Competitive inhibitors reduce the concentration of enzyme.
C) Competitive inhibitors increase the concentration of enzyme.
D) Competitive inhibitors reduce the concentration of substrate.
E) Competitive inhibitors increase the concentration of substrate.
A) Competitive inhibitors denature the enzyme.
B) Competitive inhibitors reduce the concentration of enzyme.
C) Competitive inhibitors increase the concentration of enzyme.
D) Competitive inhibitors reduce the concentration of substrate.
E) Competitive inhibitors increase the concentration of substrate.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
Chemical energy is a form of kinetic energy.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
Some enzymes have a receptor site that is other than the active site. This is also referred to as a(n) ____ site.
A) sensitive
B) allosteric
C) inhibitive
D) reversible
E) competitive
A) sensitive
B) allosteric
C) inhibitive
D) reversible
E) competitive

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
Briefly explain what is meant by the phrase "coupling of chemical reactions." Provide an example to explain the importance of this process in living organisms.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
Use your own words to explain the second law of thermodynamics and how this law applies to living organisms.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
According to the Second Law of Thermodynamics, energy cannot be created nor destroyed.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
Compare potential and kinetic energy. Give one example of kinetic energy and one example of potential energy to clarify their differences.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
Enzyme names are usually derived from the name of its substrate followed by which suffix?
A) -ase
B) -tic
C) -yst
D) -lose
E) -ator
A) -ase
B) -tic
C) -yst
D) -lose
E) -ator

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
How does the ability of ATP to transfer a phosphate group make it pivotal in the overall energy metabolism of a cell?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
A closed system does not exchange energy with its surroundings.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
In noncompetitive inhibition, the inhibitor binds to the active site of the enzyme.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
Because enzymes affect the speed of chemical reactions without being consumed, they are referred to as
A) catalysts.
B) cytochromes.
C) activation energy.
D) hydrogen acceptors.
E) transformation proteins.
A) catalysts.
B) cytochromes.
C) activation energy.
D) hydrogen acceptors.
E) transformation proteins.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
What would happen if you were to increase the temperature in an enzyme-catalyzed reaction?
A) The rate of reaction will not change.
B) The rate of reaction will increase and then level off.
C) The rate of reaction will decrease and then level off.
D) The rate of reaction will increase and then decrease rapidly.
E) The rate of reaction will decrease and then increase rapidly.
A) The rate of reaction will not change.
B) The rate of reaction will increase and then level off.
C) The rate of reaction will decrease and then level off.
D) The rate of reaction will increase and then decrease rapidly.
E) The rate of reaction will decrease and then increase rapidly.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
Explain the process of allosteric enzyme regulation. What role do allosteric regulators play in this process?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
An enzyme raises the activation energy of the reaction it catalyzes.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
Some enzymes can only function if an additional chemical component, called a cofactor, is present.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
At a constant temperature and pH, the rate of an enzymatically catalyzed reaction is inversely proportional to the enzyme concentration.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
Briefly explain how an enzyme lowers the required energy of activation for a reaction.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
FAD becomes oxidized when it accepts hydrogen atoms and their electrons.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
The cell maintains a ratio of ATP to ADP that is at the equilibrium point.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
In competitive inhibition, the inhibitor binds to the enzyme at the allosteric site.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
What would happen if all chemical processes reached equilibrium in the cell? Briefly explain why cells work to prevent a state of equilibrium.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
Most vitamins are coenzymes.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
A reversible inhibitor forms weak chemical bonds with the enzyme.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
Enzymes are highly specific and will only catalyze one or a few reactions. What would happen if most biochemical reactions were catalyzed by the same enzyme?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
A(n) exergonic reaction results in a net gain of free energy.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
A cell must expend energy to produce a concentration gradient.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck


