Deck 7: Energy and Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 7: Energy and Metabolism
1
Suppose the free energy of the reactants is greater than the free energy of the products. Such a reaction is referred to as a(n) ____.
A) entropic reaction.
B) endergonic reaction.
C) exergonic reaction.
D) catabolic reaction.
E) activation reaction.
A) entropic reaction.
B) endergonic reaction.
C) exergonic reaction.
D) catabolic reaction.
E) activation reaction.
C
2
An organism can exchange matter and energy with its surroundings. Thus, any change in an organism's energy content must be balanced by a corresponding change in the energy content of the surroundings. As such, an organism is referred to as a(n):
A) closed system.
B) open system.
C) dynamic system.
D) potential system.
E) thermally reactive system.
A) closed system.
B) open system.
C) dynamic system.
D) potential system.
E) thermally reactive system.
B
3
Figure 7-1 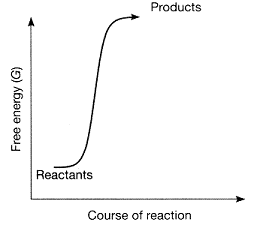 Which statement best describes the plot in the accompanying figure?
Which statement best describes the plot in the accompanying figure?
A) The figure represents an endergonic reaction.
B) The figure represents a spontaneous reaction.
C) The products have more free energy than the reactants.
D) The reactants have more free energy than the products.
E) The reaction is endergonic, and in addition, the products have more free energy than the reactants.
 Which statement best describes the plot in the accompanying figure?
Which statement best describes the plot in the accompanying figure?A) The figure represents an endergonic reaction.
B) The figure represents a spontaneous reaction.
C) The products have more free energy than the reactants.
D) The reactants have more free energy than the products.
E) The reaction is endergonic, and in addition, the products have more free energy than the reactants.
E
4
Energy that is useable and organized is classified as having ____.
A) low energy
B) low entropy
C) low enthalpy
D) high entropy
E) high energy
A) low energy
B) low entropy
C) low enthalpy
D) high entropy
E) high energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
You are out cycling and start to climb a hill. As you crest the hill, just before cycling down, what energy have you stored?
A) kinetic energy
B) thermal energy
C) chemical energy
D) electrical energy
E) potential energy
A) kinetic energy
B) thermal energy
C) chemical energy
D) electrical energy
E) potential energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
What process occurs when complex molecules are synthesized from simpler substances?
A) anabolism
B) catabolism
C) metabolism
D) condensation
E) phosphorylation
A) anabolism
B) catabolism
C) metabolism
D) condensation
E) phosphorylation

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
In a reaction in which the rate of the reverse reaction is equal to the rate of the forward reaction, a state of ____ is attained.
A) total entropy
B) enthalpy
C) thermodynamics
D) dynamic equilibrium
E) product reversibility
A) total entropy
B) enthalpy
C) thermodynamics
D) dynamic equilibrium
E) product reversibility

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
What is the ultimate source of energy for almost all living organisms?:
A) heat
B) lipids
C) the sun
D) glucose
E) carbon dioxide
A) heat
B) lipids
C) the sun
D) glucose
E) carbon dioxide

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
As you climb a flight of stairs, what type of energy are your legs in motion using?
A) kinetic energy
B) thermal energy
C) chemical energy
D) electrical energy
E) potential energy
A) kinetic energy
B) thermal energy
C) chemical energy
D) electrical energy
E) potential energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the following two chemical equations:
A) glucose + fructose → sucrose + H2O, Δ G = +27kJ/mole (or +6.5 kcal/mole)
B) glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = − 5kJ/mole (or − 1.2 kcal/mole)
What is the overall Δ G ?
A) 22 kJ/mole
B) 32 kJ/mole
C) -32 kJ/mole
D) -135 kJ/mole
E) 135 kJ/mole
A) glucose + fructose → sucrose + H2O, Δ G = +27kJ/mole (or +6.5 kcal/mole)
B) glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = − 5kJ/mole (or − 1.2 kcal/mole)
What is the overall Δ G ?
A) 22 kJ/mole
B) 32 kJ/mole
C) -32 kJ/mole
D) -135 kJ/mole
E) 135 kJ/mole

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
Every type of chemical bond contains a certain amount of energy. The total bond energy, which is essentially equivalent to the total potential energy of the system, is a quantity known as:
A) entropy.
B) enthalpy.
C) free energy.
D) kinetic energy.
E) thermodynamic energy.
A) entropy.
B) enthalpy.
C) free energy.
D) kinetic energy.
E) thermodynamic energy.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
Figure 7-1 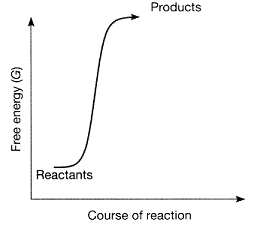 Which conclusion can be accurately derived from the accompanying figure?
Which conclusion can be accurately derived from the accompanying figure?
A) Δ S is positive.
B) Δ H equals zero.
C) Δ G is positive.
D) Δ G is negative.
E) Δ T is negative.
 Which conclusion can be accurately derived from the accompanying figure?
Which conclusion can be accurately derived from the accompanying figure?A) Δ S is positive.
B) Δ H equals zero.
C) Δ G is positive.
D) Δ G is negative.
E) Δ T is negative.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
Which best describes a catabolic reaction?
A) It involves the expenditure of energy.
B) It involves the anabolic production of complex molecules.
C) It involves the breakdown of life sustaining processes within cells.
D) It involves the breakdown of large organic molecules to simple building blocks.
E) It involves the building up of complex organic molecules from simple building blocks.
A) It involves the expenditure of energy.
B) It involves the anabolic production of complex molecules.
C) It involves the breakdown of life sustaining processes within cells.
D) It involves the breakdown of large organic molecules to simple building blocks.
E) It involves the building up of complex organic molecules from simple building blocks.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
According to the second law of thermodynamics, the amount of usable energy available to do work in the universe ____ over time.
A) crashes
B) decreases
C) increases
D) stays constant
E) exponentially increases
A) crashes
B) decreases
C) increases
D) stays constant
E) exponentially increases

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is contrary to the first law of thermodynamics?
A) When energy is used, it is irreplaceable.
B) Matter can be converted into energy.
C) The amount of energy in the universe is constant.
D) Energy can be converted from one form to another.
E) Energy can be transferred from one form to another.
A) When energy is used, it is irreplaceable.
B) Matter can be converted into energy.
C) The amount of energy in the universe is constant.
D) Energy can be converted from one form to another.
E) Energy can be transferred from one form to another.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
What unit is used to measure the thermal energy that flows from an object of a higher temperature to an object of a lower temperature?
A) kilograms
B) kilometers
C) kilowatts
D) kilocalories
E) kilojoules
A) kilograms
B) kilometers
C) kilowatts
D) kilocalories
E) kilojoules

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
Why type of energy is represented by a positive change in G ?
A) entropy
B) enthalpy
C) exergonic
D) endergonic
E) activation
A) entropy
B) enthalpy
C) exergonic
D) endergonic
E) activation

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following accurately represents the relationship between the terms anabolism, catabolism, and metabolism?
A) anabolism = catabolism
B) metabolism = catabolism
C) catabolism = anabolism + metabolism
D) anabolism = catabolism + metabolism
E) metabolism = catabolism + anabolism
A) anabolism = catabolism
B) metabolism = catabolism
C) catabolism = anabolism + metabolism
D) anabolism = catabolism + metabolism
E) metabolism = catabolism + anabolism

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
An endergonic reaction can proceed only if it absorbs:
A) randomly available free energy.
B) less free energy than is released by a coupled exergonic reaction.
C) more free energy than is released by a coupled exergonic reaction.
D) less free energy than is released by a coupled endergonic reaction.
E) the same amount of free energy that is absorbed by the enzymatic breakdown of proteins.
A) randomly available free energy.
B) less free energy than is released by a coupled exergonic reaction.
C) more free energy than is released by a coupled exergonic reaction.
D) less free energy than is released by a coupled endergonic reaction.
E) the same amount of free energy that is absorbed by the enzymatic breakdown of proteins.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
In order for a cell to maintain a high degree of order, it must constantly:
A) use energy.
B) produce energy.
C) destroy energy.
D) release energy.
E) increase energy.
A) use energy.
B) produce energy.
C) destroy energy.
D) release energy.
E) increase energy.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
Why are enzymes considered important biological catalysts?
A) Enzymes lower the entropy of a biochemical reaction.
B) Enzymes decrease the enthalpy of a biochemical reaction.
C) Enzymes increase the free energy of a biochemical reaction.
D) Enzymes supply the energy to initiate a biochemical reaction.
E) Enzymes lower the activation energy of a biochemical reaction.
A) Enzymes lower the entropy of a biochemical reaction.
B) Enzymes decrease the enthalpy of a biochemical reaction.
C) Enzymes increase the free energy of a biochemical reaction.
D) Enzymes supply the energy to initiate a biochemical reaction.
E) Enzymes lower the activation energy of a biochemical reaction.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
The transfer of electrons from one compound to another is equivalent to ____ transfer.
A) heat
B) energy
C) oxygen
D) enzymatic
E) phosphorus
A) heat
B) energy
C) oxygen
D) enzymatic
E) phosphorus

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
What form of energy is stored within the molecules of ATP?
A) kinetic
B) heat
C) potential
D) nuclear
E) light
A) kinetic
B) heat
C) potential
D) nuclear
E) light

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
Which best describes the energy of activation?
A) A type of exergonic reaction
B) A type of endergonic reaction
C) The energy required to break existing bonds
D) The catalysts needed to raise a reaction's rate
E) The enzymes required to lower a reaction's rate
A) A type of exergonic reaction
B) A type of endergonic reaction
C) The energy required to break existing bonds
D) The catalysts needed to raise a reaction's rate
E) The enzymes required to lower a reaction's rate

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a hydrogen ion acceptor?
A) ATP
B) ADP
C) FAD
D) FADH
E) NADPH
A) ATP
B) ADP
C) FAD
D) FADH
E) NADPH

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
Figure 7-2 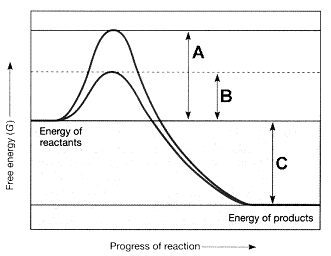 Refer to the accompanying figure. The line on the graph labeled B represents the ____.
Refer to the accompanying figure. The line on the graph labeled B represents the ____.
A) change in entropy
B) change in enthalpy
C) free energy of the reactants
D) activation energy with an enzyme
E) activation energy without an enzyme
 Refer to the accompanying figure. The line on the graph labeled B represents the ____.
Refer to the accompanying figure. The line on the graph labeled B represents the ____.A) change in entropy
B) change in enthalpy
C) free energy of the reactants
D) activation energy with an enzyme
E) activation energy without an enzyme

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements concerning ATP is FALSE?
A) It is a nucleotide.
B) It stores energy for long periods.
C) It is called the energy currency of the cell.
D) It contains phosphate groups joined in a series.
E) It contains phosphate groups joined by unstable bonds.
A) It is a nucleotide.
B) It stores energy for long periods.
C) It is called the energy currency of the cell.
D) It contains phosphate groups joined in a series.
E) It contains phosphate groups joined by unstable bonds.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
The maintenance of a high ATP to ADP ratio within cells ensures that the:
A) hydrolysis of ADP to ATP will be strongly exergonic.
B) hydrolysis of ATP to ADP will be strongly exergonic.
C) hydrolysis of ATP to ADP will be strongly endergonic.
D) hydrolysis of ADP to ATP will be an energy releasing reaction.
E) conversion of ADP to ATP will proceed spontaneously.
A) hydrolysis of ADP to ATP will be strongly exergonic.
B) hydrolysis of ATP to ADP will be strongly exergonic.
C) hydrolysis of ATP to ADP will be strongly endergonic.
D) hydrolysis of ADP to ATP will be an energy releasing reaction.
E) conversion of ADP to ATP will proceed spontaneously.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
The direction of an exergonic reaction can best be describe as moving:
A) from higher to lower entropy.
B) from lower to higher free energy.
C) from higher to lower free energy.
D) from higher to lower absolute temperature.
E) from lower to higher absolute temperature.
A) from higher to lower entropy.
B) from lower to higher free energy.
C) from higher to lower free energy.
D) from higher to lower absolute temperature.
E) from lower to higher absolute temperature.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
Select the hydrogen acceptor molecule that stores electrons in the process of photosynthesis.
A) nicotinamide adenine dinucleotide phosphate (NADP+)
B) nicotinamide adenine dinucleotide (NAD+)
C) flavin adenine dinucleotide (FAD)
D) adenine triphosphate (ATP)
E) adenine diphosphate (ADP)
A) nicotinamide adenine dinucleotide phosphate (NADP+)
B) nicotinamide adenine dinucleotide (NAD+)
C) flavin adenine dinucleotide (FAD)
D) adenine triphosphate (ATP)
E) adenine diphosphate (ADP)

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
The equation, G = H − TS , predicts that:
A) metabolism decreases proportionately to anabolism.
B) metabolism increases proportionately to catabolism.
C) as enthalpy decreases, the amount of entropy also decreases.
D) as entropy increases, the amount of free energy decreases.
E) as enthalpy increases, the amount of free energy increases.
A) metabolism decreases proportionately to anabolism.
B) metabolism increases proportionately to catabolism.
C) as enthalpy decreases, the amount of entropy also decreases.
D) as entropy increases, the amount of free energy decreases.
E) as enthalpy increases, the amount of free energy increases.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
Which chemical process occurs in which a substance loses electrons?
A) entropy
B) enthalpy
C) oxidation
D) reduction
E) anabolism
A) entropy
B) enthalpy
C) oxidation
D) reduction
E) anabolism

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
Figure 7-2 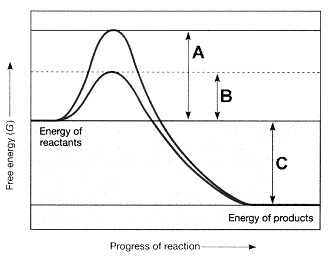 Refer to the accompanying figure. The line on the graph labeled C represents the ____.
Refer to the accompanying figure. The line on the graph labeled C represents the ____.
A) change in entropy.
B) change in enthalpy.
C) change in free energy.
D) activation energy with an enzyme.
E) activation energy without an enzyme.
 Refer to the accompanying figure. The line on the graph labeled C represents the ____.
Refer to the accompanying figure. The line on the graph labeled C represents the ____.A) change in entropy.
B) change in enthalpy.
C) change in free energy.
D) activation energy with an enzyme.
E) activation energy without an enzyme.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
Select the phosphorylation reaction.
A) glucose + fructose → sucrose + H2O
B) glucose + ATP → glucose-P + ADP
C) glucose-P + fructose → sucrose + Pi
D) glucose + glucose → maltose
E) sucrose + H2O → glucose + fructose
A) glucose + fructose → sucrose + H2O
B) glucose + ATP → glucose-P + ADP
C) glucose-P + fructose → sucrose + Pi
D) glucose + glucose → maltose
E) sucrose + H2O → glucose + fructose

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
The reaction ATP + H2O → ADP + Pi is classified as an:
A) endergonic reaction.
B) enthalpy reaction.
C) entropy reaction.
D) exergonic reaction.
E) electrochemical reaction.
A) endergonic reaction.
B) enthalpy reaction.
C) entropy reaction.
D) exergonic reaction.
E) electrochemical reaction.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
What refers to the situation in which the binding of a substrate to the enzyme causes a change in the enzyme's shape, facilitating an enzyme's function?
A) active site
B) cofactor
C) induced fit
D) activation energy
E) allosteric inhibition
A) active site
B) cofactor
C) induced fit
D) activation energy
E) allosteric inhibition

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following two chemical equations:
A) glucose + fructose → sucrose + H2O, Δ G = +27kJ/mole (or +6.5 kcal/mole)
B) glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = − 5kJ/mole (or − 1.2 kcal/mole)
The free energy change difference between the chemical equations (A) and (B) above is accomplished by:
A) a decrease in activation energy.
B) combining two endergonic reactions.
C) combining an endergonic and an exergonic reaction.
D) combining two exergonic reactions.
E) measuring the reaction rate.
A) glucose + fructose → sucrose + H2O, Δ G = +27kJ/mole (or +6.5 kcal/mole)
B) glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = − 5kJ/mole (or − 1.2 kcal/mole)
The free energy change difference between the chemical equations (A) and (B) above is accomplished by:
A) a decrease in activation energy.
B) combining two endergonic reactions.
C) combining an endergonic and an exergonic reaction.
D) combining two exergonic reactions.
E) measuring the reaction rate.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
Identify which compound has been reduced: XH2 + NAD+ → X + NADH + H+.
A) XH2
B) NAD+
C) X
D) NADH
E) H+
A) XH2
B) NAD+
C) X
D) NADH
E) H+

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
What is needed in the following reaction: ____ + Pi + energy → ADP.
A) ATP
B) H2O
C) AMP
D) cAMP
E) glucose-P
A) ATP
B) H2O
C) AMP
D) cAMP
E) glucose-P

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
What are the parts of the enzyme molecule that interact with a substrate called?
A) cofactors
B) active sites
C) reaction sites
D) enzyme product sites
E) enzyme-substrate complexes
A) cofactors
B) active sites
C) reaction sites
D) enzyme product sites
E) enzyme-substrate complexes

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
Because enzymes affect the speed of chemical reactions without being consumed, they are referred to as:
A) catalysts.
B) cytochromes.
C) activation energy.
D) hydrogen acceptors.
E) transformation proteins.
A) catalysts.
B) cytochromes.
C) activation energy.
D) hydrogen acceptors.
E) transformation proteins.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
In what method do competitive inhibitors inhibit biochemical reactions?
A) Competitive inhibitors denature the enzyme.
B) Competitive inhibitors reduce the concentration of enzyme.
C) Competitive inhibitors increase the concentration of enzyme.
D) Competitive inhibitors reduce the concentration of substrate.
E) Competitive inhibitors increase the concentration of substrate.
A) Competitive inhibitors denature the enzyme.
B) Competitive inhibitors reduce the concentration of enzyme.
C) Competitive inhibitors increase the concentration of enzyme.
D) Competitive inhibitors reduce the concentration of substrate.
E) Competitive inhibitors increase the concentration of substrate.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
Explain the process of feedback inhibition. What role do allosteric regulators play in this process?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
What is meant by the "coupling of chemical reactions"? Provide two relevant biological examples to help explain the importance of this process to living organisms.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
In the equation H = G + TS , the H stands for free energy .
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
What do hydrolases catalyze?
A) splitting water
B) oxidation-reduction reactions
C) splitting a molecule using water
D) the transfer of a phosphate group
E) reactions in which double bonds are formed
A) splitting water
B) oxidation-reduction reactions
C) splitting a molecule using water
D) the transfer of a phosphate group
E) reactions in which double bonds are formed

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
Chemical energy is a form of kinetic energy.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
Scientists usually name enzymes by adding what suffix to the name of the substrate with which the enzyme is associated?
A) -ase
B) -tic
C) -yst
D) -lose
E) -ator
A) -ase
B) -tic
C) -yst
D) -lose
E) -ator

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
Explain the second law of thermodynamics in your own words, and briefly explain how this law applies to living organisms.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
Many bacterial infections are treated with drugs, such as penicillin and sulpha drugs, that ____ the bacterium's enzyme activity.
A) inhibit
B) promote
C) decrease
D) stabilize
E) accentuate
A) inhibit
B) promote
C) decrease
D) stabilize
E) accentuate

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
A(n) closed system does not exchange energy with its surroundings.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
According to the second law of thermodynamics, energy cannot be created nor destroyed.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
Some enzymes have a receptor site that is other than the active site. This is also referred to as a(n) ____ site.
A) sensitive
B) allosteric
C) inhibitive
D) reversible
E) competitive
A) sensitive
B) allosteric
C) inhibitive
D) reversible
E) competitive

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following statements concerning enzymes is FALSE?
A) Each enzyme has an optimal temperature.
B) Each enzyme has an optimal pH.
C) Most enzymes are highly specific.
D) Some enzymes require cofactors.
E) Most enzymes are RNA molecules.
A) Each enzyme has an optimal temperature.
B) Each enzyme has an optimal pH.
C) Most enzymes are highly specific.
D) Some enzymes require cofactors.
E) Most enzymes are RNA molecules.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
Which refers to an organic, nonpolypeptide compound that binds to the apoenzyme and serves as a cofactor?
A) mineral
B) catalyst
C) substrate
D) coenzyme
E) allosteric regulator
A) mineral
B) catalyst
C) substrate
D) coenzyme
E) allosteric regulator

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
Compare and contrast potential and kinetic energy. Give two examples that clarify the differences between the two.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
You conduct an experiment in which you add increasing amounts of substrate to an enzyme solution and then measure the resulting reaction rate. You plot the rate of the reaction on the Y-axis versus substrate concentration on the X-axis. What do you conclude from your graph?
A) The reaction rate is independent of substrate concentration.
B) The reaction rate decreases with increasing substrate concentration.
C) The reaction rate is directly proportional to substrate concentration.
D) The reaction rate increases but then decreases, forming a bell-shaped curve.
E) The reaction rate increases with increasing substrate concentration up to a point, above which the rate remains constant.
A) The reaction rate is independent of substrate concentration.
B) The reaction rate decreases with increasing substrate concentration.
C) The reaction rate is directly proportional to substrate concentration.
D) The reaction rate increases but then decreases, forming a bell-shaped curve.
E) The reaction rate increases with increasing substrate concentration up to a point, above which the rate remains constant.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
What would happen if you were to increase the temperature in an enzyme-catalyzed reaction?
A) The rate of reaction will not change.
B) The rate of reaction will increase and then level off.
C) The rate of reaction will decrease and then level off.
D) The rate of reaction will increase and then decrease rapidly.
E) The rate of reaction will decrease and then increase rapidly.
A) The rate of reaction will not change.
B) The rate of reaction will increase and then level off.
C) The rate of reaction will decrease and then level off.
D) The rate of reaction will increase and then decrease rapidly.
E) The rate of reaction will decrease and then increase rapidly.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
The conversion of polysaccharides to monosaccharides is an example of a(n) anabolic reaction.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
How does the ability of ATP to transfer a phosphate group make it pivotal in the overall energy metabolism of a cell?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
An enzyme raises the activation energy of the reaction it catalyzes.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
An enzyme binds its substrate at the active site .
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
It is a fact that enzymes are highly specific and will only catalyze one or a few reactions. Can you think of a benefit that is derived from such specificity? (i.e., What would happen if most biochemical reactions were catalyzed by the same enzyme?)

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
A cell must expend energy to produce a concentration gradient.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
The cell maintains a ratio of ATP to ADP that is at the equilibrium point.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
In competitive inhibition, the inhibitor binds to the enzyme at the allosteric site.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
A(n) exergonic reaction results in a net gain of free energy.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
Most vitamins are coenzymes .
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
ATP is a type of nucleotide .
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
How is it possible for an enzyme to lower the required energy of activation for a reaction?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
Living cells maintain biochemical reactions far from equilibrium conditions. They do this by constantly manipulating the concentrations of reactants and products. Why is it important that a state far from equilibrium be maintained?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
At a constant temperature and pH, the rate of an enzymatically catalyzed reaction is inversely proportional to the enzyme concentration.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
FAD becomes oxidized when it accepts hydrogen atoms and their electrons.
__________________
__________________

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck


