Deck 2: Life, Chemistry, and Water
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 2: Life, Chemistry, and Water
1
An oxygen atom has ____ surrounding a nucleus composed of ____.
A) neutrons; electrons and protons
B) electrons; protons and neutrons
C) protons and electrons; neutrons
D) protons; neutrons and electrons
E) electrons and neutrons; protons
A) neutrons; electrons and protons
B) electrons; protons and neutrons
C) protons and electrons; neutrons
D) protons; neutrons and electrons
E) electrons and neutrons; protons
B
2
Ice floats in liquid water because there are, on average, fewer hydrogen bonds between molecules in ice than water, resulting in a lower density.
False
3
The four elements that make up more than 96.5% of the weight of living organisms are oxygen, carbon, hydrogen, and calcium.
False
4
Radioactive ____ is commonly used to treat patients with dangerously overactive thyroid glands.
A) carbon
B) radium
C) iodine
D) thallium
E) cobalt
A) carbon
B) radium
C) iodine
D) thallium
E) cobalt

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
A trace element is one found in specific organisms in ____ quantities and is ____ for normal biological functions.
A) moderate; unnecessary
B) moderate; vital
C) small; unnecessary
D) large; unnecessary
E) small; vital
A) moderate; unnecessary
B) moderate; vital
C) small; unnecessary
D) large; unnecessary
E) small; vital

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
The smallest unit that retains the chemical and physical properties of an element is a(n) ____.
A) proton
B) compound
C) molecule
D) neutron
E) atom
A) proton
B) compound
C) molecule
D) neutron
E) atom

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
Atoms with atomic numbers between lithium and neon have two energy levels.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
Prolonged iodine deficiency causes ____, a condition in which the thyroid gland enlarges so much that the front of the neck swells significantly.
A) gout
B) cancer
C) a goiter
D) anemia
E) granuloma
A) gout
B) cancer
C) a goiter
D) anemia
E) granuloma

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
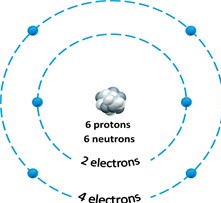 Figure 2.1
Figure 2.1Answer the question using the accompanying figure. The mass number of the atom depicted in the figure is ____.
A) 4
B) 6
C) 8
D) 12
E) 18

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
Carbon dioxide is an element.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
11
In the representation of hydrogen gas, H-H, the dash represents two electrons being shared equally.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
The substance H2O is considered to be ____.
A) both a molecule and a compound
B) a compound but not a molecule
C) neither a molecule nor a compound
D) a molecule but not a compound
E) both a molecule and an ion
A) both a molecule and a compound
B) a compound but not a molecule
C) neither a molecule nor a compound
D) a molecule but not a compound
E) both a molecule and an ion

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
Acid precipitation can have a pH as low as 3.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
Four elements, including ____, make up more than 96.5% of the mass of most living organisms.
A) sodium
B) potassium
C) phosphorus
D) nitrogen
E) calcium
A) sodium
B) potassium
C) phosphorus
D) nitrogen
E) calcium

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
Proteins in thermophiles must be stabilized by van der Waals forces, because hydrogen bonds cannot be maintained at high temperatures.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
Buffers can increase the pH of a solution when acids are added.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
The substance O2is considered to be ____.
A) both a molecule and a compound
B) a compound but not a molecule
C) neither a molecule nor a compound
D) a molecule but not a compound
E) both a molecule and an ion
A) both a molecule and a compound
B) a compound but not a molecule
C) neither a molecule nor a compound
D) a molecule but not a compound
E) both a molecule and an ion

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
Diluted acetic acid, CH3COOH, is commonly called vinegar. How many atoms of hydrogen are present in one molecule of acetic acid?
A) one
B) two
C) three
D) four
E) five
A) one
B) two
C) three
D) four
E) five

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
19
The polarity of water allows it to create a hydration layer that prevents salt from coming back out of solution after it has been dissolved.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
Hydrogen, atomic number 1, has three isotopes,1H,2H,3H.1H is comprised of one proton, one neutron, and one electron.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
Which element is likely to be chemically unreactive?
A) chlorine (seven valence electrons)
B) calcium (two valence electrons)
C) argon (eight valence electrons)
D) carbon (four valence electrons)
E) potassium (one valence electron)
A) chlorine (seven valence electrons)
B) calcium (two valence electrons)
C) argon (eight valence electrons)
D) carbon (four valence electrons)
E) potassium (one valence electron)

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
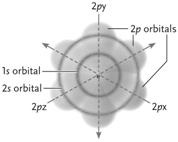 Figure 2.2
Figure 2.2Answer the question using the accompanying figure. All of the orbitals shown in the neon atom are completely filled with electrons. How many electrons does this neon atom have?
A) five
B) six
C) eight
D) 10
E) 16

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
Metallic ions such as Ca2+, Na+, and Fe3+readily form ____.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
Isotopes of the same element differ from each other in the number of ____.
A) electrons and protons
B) neutrons only
C) protons and neutrons
D) electrons only
E) protons, neutrons, and electrons
A) electrons and protons
B) neutrons only
C) protons and neutrons
D) electrons only
E) protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
A carbon atom with six protons, seven neutrons, and six electrons has a mass number of ____.
A) 6
B) 7
C) 12
D) 13
E) 19
A) 6
B) 7
C) 12
D) 13
E) 19

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the three atomic particles are charged?
A) electrons and protons
B) neutrons only
C) protons and neutrons
D) electrons only
E) protons, neutrons, and electrons
A) electrons and protons
B) neutrons only
C) protons and neutrons
D) electrons only
E) protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
The chemical linkages that exert an attractive force over the greatest distance are ____.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
Sodium has one valence electron in its third energy level. To reach a stable energy configuration, sodium will tend to____.
A) take up an electron from another atom
B) move its valence electron to the second energy shell
C) give up an electron to another atom
D) share its valence electron with another atom
E) move an electron from the second energy level to the valence shell
A) take up an electron from another atom
B) move its valence electron to the second energy shell
C) give up an electron to another atom
D) share its valence electron with another atom
E) move an electron from the second energy level to the valence shell

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
Answer the question using the accompanying figure. The atomic number of the atom depicted in the figure is ____.
A) 4
B) 6
C) 8
D) 12
E) 18
A) 4
B) 6
C) 8
D) 12
E) 18

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
Which element is most likely to accept an electron from another atom?
A) chlorine (seven valence electrons)
B) calcium (two valence electrons)
C) neon (eight valence electrons)
D) carbon (four valence electrons)
E) potassium (one valence electron)
A) chlorine (seven valence electrons)
B) calcium (two valence electrons)
C) neon (eight valence electrons)
D) carbon (four valence electrons)
E) potassium (one valence electron)

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
When the isotope14C undergoes radioactive decay, a neutron splits into an electron and a proton, with ejection of the electron. This decay produces an atom of ____.
A) iron
B) carbon
C) hydrogen
D) oxygen
E) nitrogen
A) iron
B) carbon
C) hydrogen
D) oxygen
E) nitrogen

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
Answer the question using the accompanying figure. The atom depicted in this figure can form ____ covalent bonds with another atom.
A) 0
B) 2
C) 4
D) 3
E) 6
A) 0
B) 2
C) 4
D) 3
E) 6

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
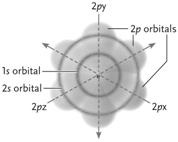 Figure 2.2
Figure 2.2Answer the question using the accompanying figure. The electrons at the lowest energy level in the neon atom depicted are found in which orbital?
A) 1 s
B) 2 s
C) 2 p x
D) 2 p y
E) 2 p z

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
The attraction between Na+cations and Cl-anions form ____ that hold the ions together in the compound NaCl.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
An orbital describes the ____ of an electron.
A) exact location
B) exact path
C) most frequent locations
D) charge
E) chemical bonds
A) exact location
B) exact path
C) most frequent locations
D) charge
E) chemical bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
14C is heavier than12C because it has ____.
A) two more electrons than12C
B) two more neutrons than12C
C) two more protons than12C
D) two more protons and two more electrons than12C
E) one more proton and one more neutron than12C
A) two more electrons than12C
B) two more neutrons than12C
C) two more protons than12C
D) two more protons and two more electrons than12C
E) one more proton and one more neutron than12C

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
Electronegativity is the tendency of an atom to attract ____ to itself in a chemical bond.
A) neutrons
B) protons
C) electrons
D) delta forces
E) polar associations
A) neutrons
B) protons
C) electrons
D) delta forces
E) polar associations

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
Chemical bonds that are formed when one atom with a partial positive charge (created from unequal sharing of electrons) is electrically attracted to another atom with a partial negative charge (also created from unequal sharing of electrons) are called ____.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
Which element is most likely to share electrons with other atoms in joint orbitals?
A) chlorine (seven valence electrons)
B) calcium (two valence electrons)
C) argon (eight valence electrons)
D) carbon (four valence electrons)
E) potassium (one valence electron)
A) chlorine (seven valence electrons)
B) calcium (two valence electrons)
C) argon (eight valence electrons)
D) carbon (four valence electrons)
E) potassium (one valence electron)

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
The chemical bonds that are formed when atoms share electrons equally are called ____.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
Which substance would have the most difficulty entering a water lattice?
A) table salt (NaCl)
B) a nonpolar molecule
C) a sodium ion
D) a proton (H+)
E) an electron
A) table salt (NaCl)
B) a nonpolar molecule
C) a sodium ion
D) a proton (H+)
E) an electron

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
In the presence of water, nonpolar associations form between molecules or regions of molecules that are ____.
A) partially charged
B) hydrophobic and hydrophilic
C) hydrophobic
D) fully charged
E) hydrophilic
A) partially charged
B) hydrophobic and hydrophilic
C) hydrophobic
D) fully charged
E) hydrophilic

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
In a molecule of methane, CH4, each hydrogen atom shares an orbital with the carbon atom. The total number of shared electrons in CH4is ____.
A) one
B) two
C) four
D) five
E) eight
A) one
B) two
C) four
D) five
E) eight

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
A polar covalent bond would be most likely to form between ____.
A) atoms with different electronegativities
B) cations and anions
C) atoms with partial positive and partial negative charges
D) atoms with filled valence shells
E) atoms of the same element
A) atoms with different electronegativities
B) cations and anions
C) atoms with partial positive and partial negative charges
D) atoms with filled valence shells
E) atoms of the same element

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
A mixture of vegetable oil and water will separate into layers because oil is ____ and forms ____.
A) hydrophobic; nonpolar associations
B) hydrophilic; nonpolar associations
C) hydrophilic; polar associations
D) hydrophobic; polar associations
E) hydrophobic; ionic associations
A) hydrophobic; nonpolar associations
B) hydrophilic; nonpolar associations
C) hydrophilic; polar associations
D) hydrophobic; polar associations
E) hydrophobic; ionic associations

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
Which type of chemical linkage is the weakest?
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
The hydrogen-bond lattice causes water to have an unusually ____ specific heat, an unusually ____ heat of vaporization, and an unusually ____ density in solid form.
A) high; high; high
B) low; low; low
C) high; low; high
D) high; high; low
E) low; low; high
A) high; high; high
B) low; low; low
C) high; low; high
D) high; high; low
E) low; low; high

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
Water has an important stabilizing effect on temperature in living organisms and their environments because as water absorbs heat, much of the energy is used to ____ instead of raising the temperature.
A) create hydrogen bonds
B) create covalent bonds
C) break surface tension
D) break hydrogen bonds
E) create hydration layers
A) create hydrogen bonds
B) create covalent bonds
C) break surface tension
D) break hydrogen bonds
E) create hydration layers

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
 Figure 2.4
Figure 2.4The water strider shown in the figure above is able to stand on water because of the ____ of water.
A) covalent bonds
B) surface tension
C) van der Waals forces
D) density
E) hydration layer

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
Water has an unusually high boiling point for its molecular weight because water molecules ____.
A) are very dense
B) get much heavier as they are heated
C) are held to each other by hydrogen bonds
D) are held together by covalent bonds
E) form hydration layers
A) are very dense
B) get much heavier as they are heated
C) are held to each other by hydrogen bonds
D) are held together by covalent bonds
E) form hydration layers

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
A molecule of water in the middle of a chunk of ice will usually have ____ hydrogen bonds with other water molecules.
A) two
B) three
C) 3.4
D) four
E) six
A) two
B) three
C) 3.4
D) four
E) six

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
When water molecules exposed to the air form hydrogen bonds between adjacent water molecules below and beside them, molecules in the upper layer become more resistant to separating from those underneath. This property of water is known as ____.
A) cohesion
B) adhesion
C) a hydration layer
D) a water lattice
E) surface tension
A) cohesion
B) adhesion
C) a hydration layer
D) a water lattice
E) surface tension

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
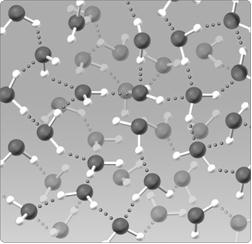 Figure 2.5
Figure 2.5The water lattice illustrated in the figure above forms as a result of ____ between water molecules.
A) covalent bonds
B) hydrogen bonds
C) nonpolar interactions
D) ionic bonds
E) van der Walls forces

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
In contrast to ionic bonds, covalent bonds ____.
A) hold atoms together
B) have distinct, three-dimensional forms
C) transfer electrons from one atom to another
D) are rarely broken
E) are transient
A) hold atoms together
B) have distinct, three-dimensional forms
C) transfer electrons from one atom to another
D) are rarely broken
E) are transient

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
Multiple hydrogen bonds together stabilize proteins into a spiral structure called a ____.
A) water lattice
B) alpha helix
C) chemical groups
D) delta minus
E) delta plus
A) water lattice
B) alpha helix
C) chemical groups
D) delta minus
E) delta plus

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
The formation and breaking of bonds between atoms requires ____.
A) a chemical reaction
B) van der Walls forces
C) partial charges
D) an empty valence shell
E) an enzyme
A) a chemical reaction
B) van der Walls forces
C) partial charges
D) an empty valence shell
E) an enzyme

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
Molecules such as H-H and O=O are held together by ____.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
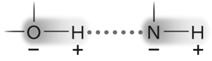 Figure 2.3
Figure 2.3Answer the question using the accompanying figure. The molecule shown is held together by ____.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
How many calories, as defined in chemistry, are in one calorie, which is the unit used to quantify the amount of energy in the food we eat?
A) 10
B) 100
C) 1,000
D) 10,000
E) 100,000
A) 10
B) 100
C) 1,000
D) 10,000
E) 100,000

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
Geckos are able to cling to vertical walls due to ____.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
When added to water at neutral pH (7.0), an acid will ____.
A) act as a proton donor, raising the pH of the solution
B) act as a proton acceptor, raising the pH of the solution
C) act as a proton donor, lowering the pH of the solution
D) act as a proton acceptor, lowering the pH of the solution
E) do nothing since the aqueous solution is neutral
A) act as a proton donor, raising the pH of the solution
B) act as a proton acceptor, raising the pH of the solution
C) act as a proton donor, lowering the pH of the solution
D) act as a proton acceptor, lowering the pH of the solution
E) do nothing since the aqueous solution is neutral

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
Solution A has a pH of 6 and solution B has a pH of 8. Therefore, ____.
A) A has 10 times greater H+concentration than B.
B) B has 10 times greater H+concentration than A.
C) A has 100 times greater H+concentration than B.
D) B has 100 times greater H+concentration than A.
E) A has 1,000 times greater H+concentration than B.
A) A has 10 times greater H+concentration than B.
B) B has 10 times greater H+concentration than A.
C) A has 100 times greater H+concentration than B.
D) B has 100 times greater H+concentration than A.
E) A has 1,000 times greater H+concentration than B.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
Biological membranes are held together mainly by ____.
A) hydrogen bonds between lipid molecules
B) hydration layers over lipid molecules
C) exclusion of the nonpolar regions of lipids by water
D) hydrogen bonds between water molecules
E) surface tension at the interface between layers of water molecules
A) hydrogen bonds between lipid molecules
B) hydration layers over lipid molecules
C) exclusion of the nonpolar regions of lipids by water
D) hydrogen bonds between water molecules
E) surface tension at the interface between layers of water molecules

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
Pure water has a pH of 7.0, therefore, ____.
A) [H+]-]
B) [H+] = [OH-]
C) [H+] = 0
D) [OH-] = 0
E) [H+]>[OH-]
A) [H+]-]
B) [H+] = [OH-]
C) [H+] = 0
D) [OH-] = 0
E) [H+]>[OH-]

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
The most common isotope of carbon has an atomic number of 6 and a mass number of 12, while the most common isotope of oxygen has an atomic number of 8 and a mass number of 16. A molecule of CO2made up of these common isotopes has a molecular weight of ____.
A) 28
B) 44
C) 56
D) 14
E) 22
A) 28
B) 44
C) 56
D) 14
E) 22

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
High levels of carbon dioxide in the atmosphere are causing ____.
A) the pH of the ocean to increase
B) the pH of the ocean to decrease
C) the natural buffers in the ocean to die
D) increased calcification of the coral reefs
E) increased biodiversity in coral reefs
A) the pH of the ocean to increase
B) the pH of the ocean to decrease
C) the natural buffers in the ocean to die
D) increased calcification of the coral reefs
E) increased biodiversity in coral reefs

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
Avogadro's number represents the ____.
A) number of grams in a mole of substance
B) number of moles in a gram of substance
C) number of atoms in one gram of substance
D) atomic weight of an atom divided by the weight of an atom of that element
E) weight of an atom of an element divided by the atomic weight of that element
A) number of grams in a mole of substance
B) number of moles in a gram of substance
C) number of atoms in one gram of substance
D) atomic weight of an atom divided by the weight of an atom of that element
E) weight of an atom of an element divided by the atomic weight of that element

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
Seawater typically is ____.
A) highly basic
B) neutral
C) somewhat basic
D) somewhat acidic
E) highly basic
A) highly basic
B) neutral
C) somewhat basic
D) somewhat acidic
E) highly basic

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
A pH of 6 is ____ times more ____ than a pH of 2.
A) 3; acidic
B) 4; acidic
C) 3; basic
D) 10,000; basic
E) 40; basic
A) 3; acidic
B) 4; acidic
C) 3; basic
D) 10,000; basic
E) 40; basic

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
A ____ is formed when a ____ is dissolved in a ____.
A) solution; solute; solvent
B) solute; solvent; solution
C) solution; solvent; solute
D) solvent; solution; solute
E) solvent; solute; solution
A) solution; solute; solvent
B) solute; solvent; solution
C) solution; solvent; solute
D) solvent; solution; solute
E) solvent; solute; solution

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
When added to water, a base will act as a(n) ____ and cause the pH of the solution to ____.
A) proton acceptor; rise
B) proton donor; rise
C) proton acceptor; fall
D) proton donor; fall
E) acid; fall
A) proton acceptor; rise
B) proton donor; rise
C) proton acceptor; fall
D) proton donor; fall
E) acid; fall

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
Ethanol, the alcohol found in wine and beer, has the molecular formula CH3CH2OH. What is the molecular weight of ethanol if the atomic weight of C=12, H=1, and O=16?
A) 29 g/mol
B) 30 g/mol
C) 34 g/mol
D) 45 g/mol
E) 46 g/mol
A) 29 g/mol
B) 30 g/mol
C) 34 g/mol
D) 45 g/mol
E) 46 g/mol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
When salt dissolves in water, the water molecules form ____ around the Na+and Cl-ions.
A) covalent bonds
B) hydration layers
C) nonpolar interactions
D) membranes
E) ionic bonds
A) covalent bonds
B) hydration layers
C) nonpolar interactions
D) membranes
E) ionic bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
Lemon juice has a pH of 2.0, therefore, ____.
A) [H+]-]
B) [H+] = [OH-]
C) [H+] = 0
D) [OH-] = 0
E) [H+]>[OH-]
A) [H+]-]
B) [H+] = [OH-]
C) [H+] = 0
D) [OH-] = 0
E) [H+]>[OH-]

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
Most pH buffers are ____.
A) strong acids
B) weak acids or weak bases
C) weak acids
D) strong bases
E) strong acids or strong bases
A) strong acids
B) weak acids or weak bases
C) weak acids
D) strong bases
E) strong acids or strong bases

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
Consider the equilibrium established in the carbonic acid-bicarbonate buffer system, which maintains pH balance in mammalian blood:
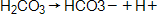
During hypoventilation, breathing rate decreases, and therefore elimination of CO2 during exhalation decreases. How is optimal blood pH maintained when acid levels increase in our blood from hypoventilating?
A) Excess H+ from the acid reacts with H2CO3to decrease pH level.
B) Excess H+ from the acid reacts with H2CO3to increase pH level.
C) Excess H+ from the acid reacts with H2CO3to maintain pH level.
D) Excess H+ from the acid reacts with HCO3- to increase pH level.
E) Excess H+ from the acid reacts with HCO3- to maintain pH level.
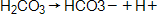
During hypoventilation, breathing rate decreases, and therefore elimination of CO2 during exhalation decreases. How is optimal blood pH maintained when acid levels increase in our blood from hypoventilating?
A) Excess H+ from the acid reacts with H2CO3to decrease pH level.
B) Excess H+ from the acid reacts with H2CO3to increase pH level.
C) Excess H+ from the acid reacts with H2CO3to maintain pH level.
D) Excess H+ from the acid reacts with HCO3- to increase pH level.
E) Excess H+ from the acid reacts with HCO3- to maintain pH level.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
When sugar dissolves in water, water is acting as a ____ and the sugar molecules are acting as ____.
A) solution; solvents
B) solute; solutions
C) solvent; solutes
D) solute; solvents
E) solvent; solutions
A) solution; solvents
B) solute; solutions
C) solvent; solutes
D) solute; solvents
E) solvent; solutions

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
Without ____, living organisms would often experience major changes in pH in their cells.
A) buffers
B) acids
C) surface tension
D) nonpolar bonds
E) bases
A) buffers
B) acids
C) surface tension
D) nonpolar bonds
E) bases

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
Water has a molecular weight of 18 g per mole, and glucose has a molecular weight of 180 g per mole. Which masses of water and glucose would have an approximately equal number of molecules?
A) 1 g of water and 180 g of glucose
B) 90 g of water and 9 g of glucose
C) 180 g of water and 1 g of glucose
D) 9 g of water and 90 g of glucose
E) 90 g of water and 90 g of glucose
A) 1 g of water and 180 g of glucose
B) 90 g of water and 9 g of glucose
C) 180 g of water and 1 g of glucose
D) 9 g of water and 90 g of glucose
E) 90 g of water and 90 g of glucose

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
In water, NaOH almost completely separates into Na+and OH-ions. Thus, NaOH is _____.
A) a strong acid
B) a strong base
C) a weak acid
D) a weak base
E) neutral
A) a strong acid
B) a strong base
C) a weak acid
D) a weak base
E) neutral

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck


