Exam 2: Life, Chemistry, and Water
Exam 1: Introduction to Biological Concepts and Research86 Questions
Exam 2: Life, Chemistry, and Water87 Questions
Exam 3: Biological Molecules: the Carbon Compounds of Life86 Questions
Exam 4: Cells87 Questions
Exam 5: Membranes and Transport88 Questions
Exam 6: Energy, Enzymes, and Biological Reactions87 Questions
Exam 7: Cellular Respiration: Harvesting Chemical Energy88 Questions
Exam 8: Photosynthesis83 Questions
Exam 9: Cell Communication87 Questions
Exam 10: Cell Division and Mitosis88 Questions
Exam 11: Meiosis: the Cellular Basis of Sexual Reproduction80 Questions
Exam 12: Mendel, Genes, and Inheritance79 Questions
Exam 13: Genes, Chromosomes, and Human Genetics92 Questions
Exam 14: Dna Structure, Replication, and Organization79 Questions
Exam 15: Gene Expression: From Dna to Protein83 Questions
Exam 16: Regulation of Gene Expression84 Questions
Exam 17: Bacterial and Viral Genetics85 Questions
Exam 18: Dna Technology: Making and Using Genetically Altered Organisms, and Other Applications90 Questions
Exam 19: Genomes and Proteomes81 Questions
Exam 20: The Development of Evolutionary Thought92 Questions
Exam 21: Microevolution: Genetic Changes Within Populations88 Questions
Exam 22: Speciation89 Questions
Exam 23: Paleobiology and Macroevolution87 Questions
Exam 24: Systematic Biology: Phylogeny and Classification95 Questions
Exam 25: The Origin of Life86 Questions
Exam 26: Prokaryotes and Viruses86 Questions
Exam 27: Protists90 Questions
Exam 28: Seedless Plants88 Questions
Exam 29: Seed Plants90 Questions
Exam 30: Fungi88 Questions
Exam 31: Animal Phylogeny, Acoelomates, and Protostomes95 Questions
Exam 32: Deuterostomes: Vertebrates and Their Closest Relatives93 Questions
Exam 33: The Plant Body90 Questions
Exam 34: Transport in Plants94 Questions
Exam 35: Plant Nutrition85 Questions
Exam 36: Reproduction and Development in Flowering Plants89 Questions
Exam 37: Plant Signals and Responses to the Environment90 Questions
Exam 38: Introduction to Animal Organization and Physiology87 Questions
Exam 39: Information Flow and the Neuron88 Questions
Exam 40: Nervous Systems88 Questions
Exam 41: Sensory Systems87 Questions
Exam 42: The Endocrine System94 Questions
Exam 43: Muscles, Bones, and Body Movements87 Questions
Exam 44: The Circulatory System87 Questions
Exam 45: Defenses Against Disease83 Questions
Exam 46: Gas Exchange: the Respiratory System87 Questions
Exam 47: Digestive Systems and Animal Nutrition92 Questions
Exam 48: Regulating the Internal Environment: Osmoregulation, Excretion, and Thermoregulation88 Questions
Exam 49: Animal Reproduction76 Questions
Exam 50: Animal Development88 Questions
Exam 51: Ecology and the Biosphere88 Questions
Exam 52: Population Ecology92 Questions
Exam 53: Population Interactions and Community Ecology89 Questions
Exam 54: Ecosystems90 Questions
Exam 55: Biodiversity and Conservation Biology89 Questions
Exam 56: Animal Behavior87 Questions
Select questions type
 Figure 2.4
The water strider shown in the figure above is able to stand on water because of the ____ of water.
Figure 2.4
The water strider shown in the figure above is able to stand on water because of the ____ of water.
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
B
Ice floats in liquid water because there are, on average, fewer hydrogen bonds between molecules in ice than water, resulting in a lower density.
Free
(True/False)
4.9/5  (37)
(37)
Correct Answer:
False
Which element is likely to be chemically unreactive?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
C
Ethanol, the alcohol found in wine and beer, has the molecular formula CH3CH2OH. What is the molecular weight of ethanol if the atomic weight of C=12, H=1, and O=16?
(Multiple Choice)
4.8/5  (24)
(24)
In water, NaOH almost completely separates into Na+and OH-ions. Thus, NaOH is _____.
(Multiple Choice)
4.8/5  (36)
(36)
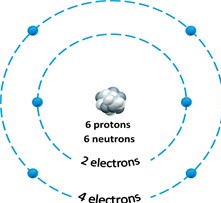 Figure 2.1
-Answer the question using the accompanying figure. The atomic number of the atom depicted in the figure is ____.
Figure 2.1
-Answer the question using the accompanying figure. The atomic number of the atom depicted in the figure is ____.
(Multiple Choice)
5.0/5  (32)
(32)
When salt dissolves in water, the water molecules form ____ around the Na+and Cl-ions.
(Multiple Choice)
4.7/5  (28)
(28)
The formation and breaking of bonds between atoms requires ____.
(Multiple Choice)
4.8/5  (23)
(23)
Diluted acetic acid, CH3COOH, is commonly called vinegar. How many atoms of hydrogen are present in one molecule of acetic acid?
(Multiple Choice)
4.8/5  (28)
(28)
The polarity of water allows it to create a hydration layer that prevents salt from coming back out of solution after it has been dissolved.
(True/False)
4.7/5  (31)
(31)
Which substance would have the most difficulty entering a water lattice?
(Multiple Choice)
4.9/5  (41)
(41)
How many calories, as defined in chemistry, are in one calorie, which is the unit used to quantify the amount of energy in the food we eat?
(Multiple Choice)
4.8/5  (44)
(44)
Which element is most likely to share electrons with other atoms in joint orbitals?
(Multiple Choice)
4.9/5  (35)
(35)
Why is iodine considered a trace element, and what is its biological function in humans?
(Essay)
4.8/5  (27)
(27)
Hydrogen, atomic number 1, has three isotopes,1H,2H,3H.1H is comprised of one proton, one neutron, and one electron.
(True/False)
4.8/5  (37)
(37)
For each of the following situations, choose the correct type of chemical bond. Some choices may be used more than once.
Correct Answer:
Premises:
Responses:
(Matching)
4.7/5  (41)
(41)
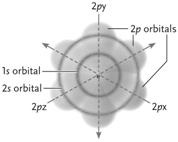 Figure 2.2
-
Answer the question using the accompanying figure. All of the orbitals shown in the neon atom are completely filled with electrons. How many electrons does this neon atom have?
Figure 2.2
-
Answer the question using the accompanying figure. All of the orbitals shown in the neon atom are completely filled with electrons. How many electrons does this neon atom have?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 1 - 20 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)