Deck 11: Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 11: Liquids and Solids
1
Which of the following phase transitions are considered exothermic ?
I. Freezing
II. Condensation
III. Deposition
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. Freezing
II. Condensation
III. Deposition
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
All of these
2
Which phase transition listed below would represent the transition from the solid state to gaseous vapor?
A) Condensation
B) Melting
C) Deposition
D) Sublimation
E) Evaporation
A) Condensation
B) Melting
C) Deposition
D) Sublimation
E) Evaporation
Sublimation
3
Consider liquid state molecules on the submicroscopic scale. Which statement below is true regarding the relative magnitudes of the forces of attraction and forces of disruption among liquid molecules?
A) The forces of attraction are much greater than the kinetic energy for liquid molecules.
B) The forces of attraction and kinetic energy are comparable for liquid molecules.
C) The kinetic energy dominates over the forces of attraction for liquid molecules.
D) A direct comparison between these relative forces cannot be made.
A) The forces of attraction are much greater than the kinetic energy for liquid molecules.
B) The forces of attraction and kinetic energy are comparable for liquid molecules.
C) The kinetic energy dominates over the forces of attraction for liquid molecules.
D) A direct comparison between these relative forces cannot be made.
The forces of attraction and kinetic energy are comparable for liquid molecules.
4
Exhibit 11-1 The heating curve below is needed for the following question(s). 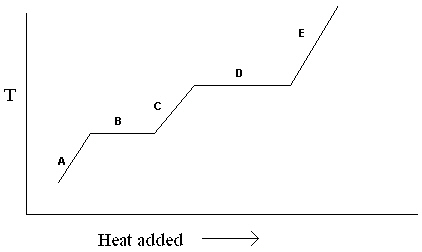
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment that corresponds to the heat of fusion?
A) A
B) B
C) C
D) D
E) E

Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment that corresponds to the heat of fusion?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 11-1 The heating curve below is needed for the following question(s). 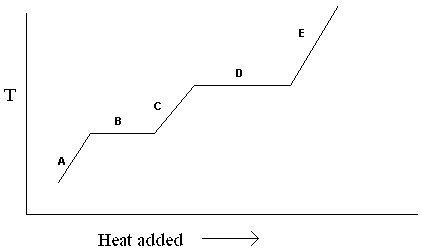
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment that corresponds to the heat of vaporization?
A) A
B) B
C) C
D) D
E) E

Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment that corresponds to the heat of vaporization?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
If the energy of intermolecular attractions are much less than the average kinetic energy of the molecules, what is the physical state of the substance?
A) solid
B) liquid
C) gas
D) plasma
E) Its state cannot be predicted
A) solid
B) liquid
C) gas
D) plasma
E) Its state cannot be predicted

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
A slush of liquid water and ice crystals is at equilibrium at 0 ° C. If a small amount of heat is removed so that more ice is formed, the system will experience:
A) a temperature rise since crystallization of ice is an exothermic process.
B) a change in vapor pressure over the mixture.
C) a temperature lowering since more ice produces a colder system.
D) a change in pressure that is proportional to the heat.
E) no temperature change.
A) a temperature rise since crystallization of ice is an exothermic process.
B) a change in vapor pressure over the mixture.
C) a temperature lowering since more ice produces a colder system.
D) a change in pressure that is proportional to the heat.
E) no temperature change.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following phase changes is an exothermic event?
I. Sublimation
II. Condensing
III. Melting
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. Sublimation
II. Condensing
III. Melting
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
Which phase change listed below represents the transition from the gaseous state to the liquid state?
A) Condensation
B) Deposition
C) Evaporation
D) Freezing
E) Sublimation
A) Condensation
B) Deposition
C) Evaporation
D) Freezing
E) Sublimation

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 11-2 The phase diagram below is needed for the following question(s). 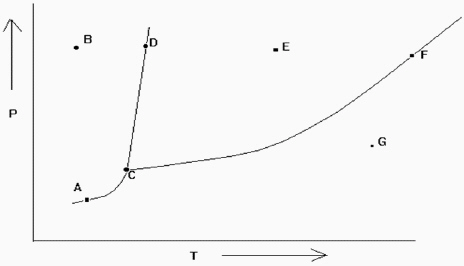
Refer to Exhibit 11-2. If one is at position A and the pressure is increased, which statement below is true?
A) One moves from a position of gas-liquid equilibrium to one in the liquid phase.
B) One moves from the gas phase to the liquid phase.
C) One moves from a position of gas-solid equilibrium to one in the liquid phase.
D) One moves from a position of gas-liquid equilibrium to one in the gas phase.
E) One moves from a position of gas-solid equilibrium to one in the solid phase.

Refer to Exhibit 11-2. If one is at position A and the pressure is increased, which statement below is true?
A) One moves from a position of gas-liquid equilibrium to one in the liquid phase.
B) One moves from the gas phase to the liquid phase.
C) One moves from a position of gas-solid equilibrium to one in the liquid phase.
D) One moves from a position of gas-liquid equilibrium to one in the gas phase.
E) One moves from a position of gas-solid equilibrium to one in the solid phase.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
Given the three statements below, pick the best answer.
I. The normal boiling point of a liquid is the temperature at which its vapor pressure is 1 atm.
II. Water always boils at 100 ° C.
III. The vapor pressure of a liquid increases with increasing temperature.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only III is true
I. The normal boiling point of a liquid is the temperature at which its vapor pressure is 1 atm.
II. Water always boils at 100 ° C.
III. The vapor pressure of a liquid increases with increasing temperature.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only III is true

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 11-1 The heating curve below is needed for the following question(s). 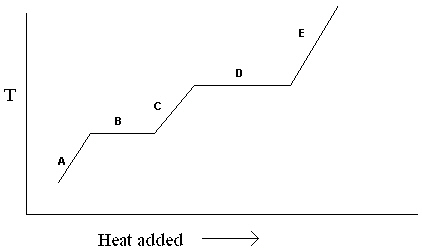
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where liquid and gas may be present?
A) A
B) B
C) C
D) D
E) E

Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where liquid and gas may be present?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
At room temperature chlorine is a gas, bromine is a liquid, and iodine is a solid. All three exist as diatomic molecules at this temperature. The intermolecular attractions in these substances increase in the order:
A) I2 < Br2 < Cl2
B) Br2 < I2 < Cl2
C) Cl2 < Br2 < I2
D) I2 < Cl2 < Br2
E) none of these
A) I2 < Br2 < Cl2
B) Br2 < I2 < Cl2
C) Cl2 < Br2 < I2
D) I2 < Cl2 < Br2
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 11-1 The heating curve below is needed for the following question(s). 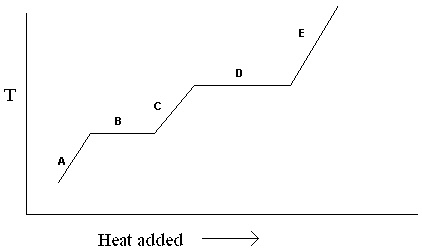
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where only solid is present?
A) A
B) B
C) C
D) D
E) E

Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where only solid is present?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 11-1 The heating curve below is needed for the following question(s). 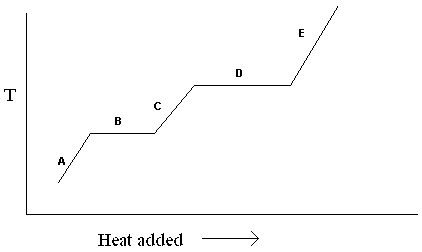
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where only liquid is present?
A) A
B) B
C) C
D) D
E) E

Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where only liquid is present?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following phase changes listed below would be considered endothermic ?
I. Sublimation
II. Deposition
III. Condensation
A) I only
B) II only
C) I and II
D) I and III
E) All of these
I. Sublimation
II. Deposition
III. Condensation
A) I only
B) II only
C) I and II
D) I and III
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
What is the transition from the gaseous phase to the solid phase?
A) Condensing
B) Deposition
C) Evaporation
D) Melting
E) Sublimation
A) Condensing
B) Deposition
C) Evaporation
D) Melting
E) Sublimation

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
For most substances, the enthalpy of the phase changes, D H sublimation, D H fusion, and D H vaporization, increase in the order:
A) D H sublimation D H fusion D H vaporization
B) D H fusion D H sublimation D H vaporization
C) D H fusion D H vaporization D H sublimation
D) D H vaporization D H fusion D H sublimation
E) D H vaporization D H sublimation D H fusion
A) D H sublimation D H fusion D H vaporization
B) D H fusion D H sublimation D H vaporization
C) D H fusion D H vaporization D H sublimation
D) D H vaporization D H fusion D H sublimation
E) D H vaporization D H sublimation D H fusion

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the three states of matter have much greater kinetic energy than their forces of attraction?
I. Solids
II. Liquids
III. Gases
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. Solids
II. Liquids
III. Gases
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
The temperature at which solid is in equilibrium with its vapor is called
A) the melting point
B) the freezing point
C) the boiling point
D) the sublimation point
E) none of these
A) the melting point
B) the freezing point
C) the boiling point
D) the sublimation point
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
Exhibit 11-2 The phase diagram below is needed for the following question(s). 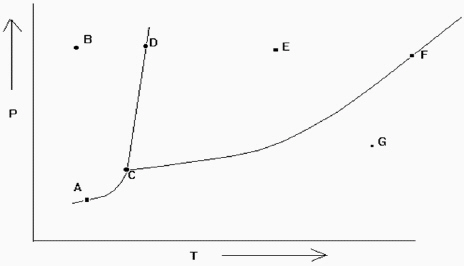
Refer to Exhibit 11-2. If one is at position F and the pressure is increased, which statement below is true?
A) One moves from a position of gas-liquid equilibrium to one in the liquid phase.
B) One moves from a position of gas-solid equilibrium to one in the liquid phase.
C) One moves from the gas phase to the liquid phase.
D) One moves from a position of gas-liquid equilibrium to one in the gas phase.
E) none of these

Refer to Exhibit 11-2. If one is at position F and the pressure is increased, which statement below is true?
A) One moves from a position of gas-liquid equilibrium to one in the liquid phase.
B) One moves from a position of gas-solid equilibrium to one in the liquid phase.
C) One moves from the gas phase to the liquid phase.
D) One moves from a position of gas-liquid equilibrium to one in the gas phase.
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 11-2 The phase diagram below is needed for the following question(s). 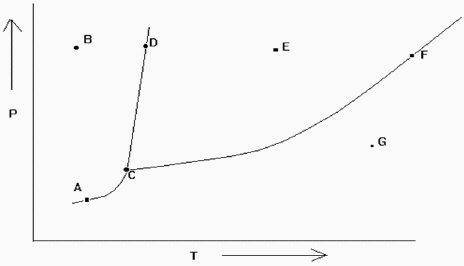
Refer to Exhibit 11-2. At which of the lettered points on the phase diagram is more than one phase present?
A) C only
B) A, D, and F only
C) D and F only
D) A, C, D, and F only
E) none of these

Refer to Exhibit 11-2. At which of the lettered points on the phase diagram is more than one phase present?
A) C only
B) A, D, and F only
C) D and F only
D) A, C, D, and F only
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
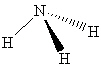
What intermolecular force(s) of interaction is(are) possible for a molecule of NH₃shown below?
A) London dispersion
B) Dipole-dipole
C) Hydrogen bonding
D) a and b
E) all of these

A) London dispersion
B) Dipole-dipole
C) Hydrogen bonding
D) a and b
E) all of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 11-2 The phase diagram below is needed for the following question(s). 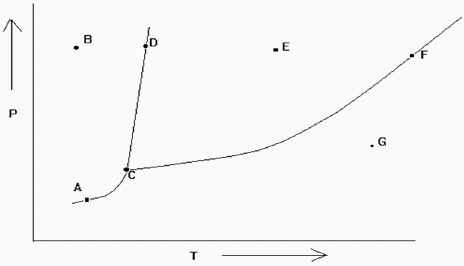
Refer to Exhibit 11-2. If the temperature and pressure are such that the substance is at point D in the diagram, a slight increase in temperature would cause what type of change?
A) change a solid into a liquid
B) change a liquid into a solid
C) go from a solid-liquid equilibrium to a liquid
D) go from a solid-liquid equilibrium to a solid
E) go from a liquid-gas equilibrium to a gas

Refer to Exhibit 11-2. If the temperature and pressure are such that the substance is at point D in the diagram, a slight increase in temperature would cause what type of change?
A) change a solid into a liquid
B) change a liquid into a solid
C) go from a solid-liquid equilibrium to a liquid
D) go from a solid-liquid equilibrium to a solid
E) go from a liquid-gas equilibrium to a gas

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
What intermolecular force(s) of attraction is(are) present between two molecules of ethanol shown below?
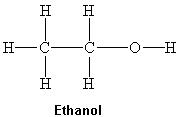
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these

I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
What intermolecular force(s) of interaction is(are) present between two molecules of dimethyl ether, CH3OCH3?
I. London dispersion forces
II. Dipole-dipole forces
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. London dispersion forces
II. Dipole-dipole forces
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
Formaldehyde is a colorless gas but when dissolved in water can be used in embalming fluids. Which intermolecular force(s) is(are) present among formaldehyde molecules shown below?
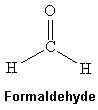
A) London dispersion
B) Dipole-dipole
C) Hydrogen bonding
D) a and b
E) all of these

A) London dispersion
B) Dipole-dipole
C) Hydrogen bonding
D) a and b
E) all of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
What intermolecular force(s) of attraction is(are) present between two molecules of ethane shown below?
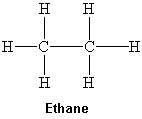
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these

I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 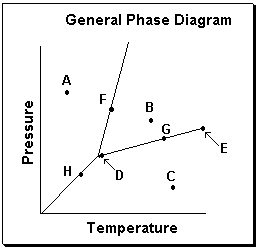
Refer to Exhibit 11-3. What phase transition would occur upon moving from point B to point C in the phase diagram above?
A) Condensation
B) Evaporation
C) Freezing
D) Melting
E) Sublimation

Refer to Exhibit 11-3. What phase transition would occur upon moving from point B to point C in the phase diagram above?
A) Condensation
B) Evaporation
C) Freezing
D) Melting
E) Sublimation

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following molecules can form hydrogen bonds ?
I. CH3NH2
II. CH3OH
III. CH3F
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. CH3NH2
II. CH3OH
III. CH3F
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 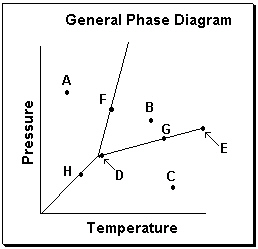
Refer to Exhibit 11-3. Which temperature-pressure point on this diagram represents conditions in which the solid phase is present in equilibrium with the liquid phase?
A) A
B) B
C) C
D) F
E) G

Refer to Exhibit 11-3. Which temperature-pressure point on this diagram represents conditions in which the solid phase is present in equilibrium with the liquid phase?
A) A
B) B
C) C
D) F
E) G

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
Which intermolecular forces of attraction represents an interaction between two molecules resulting from opposites charges (partial positive and partial negative interactions)?
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
What intermolecular force(s) of attraction is(are) present between two molecules of CH2F2?
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 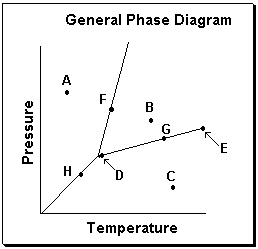
Refer to Exhibit 11-3. What phase transition would occur upon moving from point B to point A on the phase diagram above?
A) Condensation
B) Evaporation
C) Freezing
D) Melting
E) Sublimation

Refer to Exhibit 11-3. What phase transition would occur upon moving from point B to point A on the phase diagram above?
A) Condensation
B) Evaporation
C) Freezing
D) Melting
E) Sublimation

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 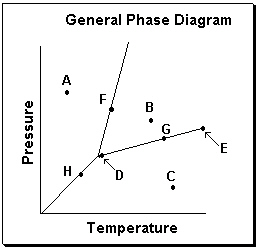
Refer to Exhibit 11-3. Which transition is related to sublimation in the phase diagram above?
A) Moving from point A to point B
B) Moving from point B to point A
C) Moving from point B to point C
D) Moving from point C to point B
E) None of these.

Refer to Exhibit 11-3. Which transition is related to sublimation in the phase diagram above?
A) Moving from point A to point B
B) Moving from point B to point A
C) Moving from point B to point C
D) Moving from point C to point B
E) None of these.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
What intermolecular force(s) is(are) present among molecules of HBr?
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following molecules can form hydrogen bonds as a pure liquid?
A)
B)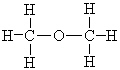
C)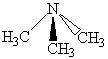
D) a and b
E) all of these
A)

B)

C)

D) a and b
E) all of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 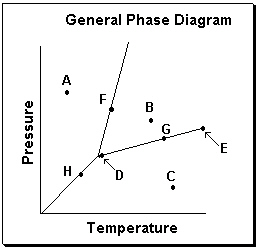
Refer to Exhibit 11-3. What temperature-pressure point on the General Phase Diagram above represents the Critical Point ?
A) D
B) E
C) F
D) G
E) H

Refer to Exhibit 11-3. What temperature-pressure point on the General Phase Diagram above represents the Critical Point ?
A) D
B) E
C) F
D) G
E) H

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
Exhibit 11-2 The phase diagram below is needed for the following question(s). 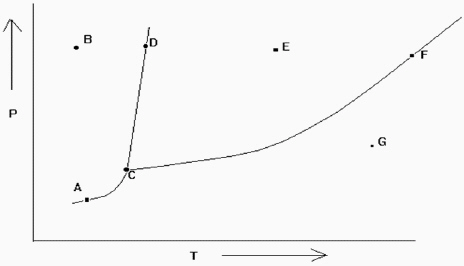
Refer to Exhibit 11-2. Which point denotes the place where all the three phases are in equilibrium at the same time?
A) A
B) B
C) C
D) D
E) E

Refer to Exhibit 11-2. Which point denotes the place where all the three phases are in equilibrium at the same time?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following substances have London Dispersion Forces of attraction?
I. He
II. CH4
III. H2O
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. He
II. CH4
III. H2O
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following forces between atoms or ions or molecules is the strongest on a per mole basis?
A) Force holding one sheet of graphite to another
B) Covalent bond
C) Dipole-dipole force
D) Hydrogen bond
E) Dispersion force
A) Force holding one sheet of graphite to another
B) Covalent bond
C) Dipole-dipole force
D) Hydrogen bond
E) Dispersion force

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
Arrange the following molecules in order of increasing boiling point. H2O2, HCl and O2
A) (lowest bp) H2O2 < HCl < O2 (highest bp)
B) (lowest bp) O2 < HCl < H2O2 (highest bp)
C) (lowest bp) HCl < H2O2 < O2 (highest bp)
D) (lowest bp) HCl < O2 < H2O2 (highest bp)
E) (lowest bp) O2 < H2O2 < HCl (highest bp)
A) (lowest bp) H2O2 < HCl < O2 (highest bp)
B) (lowest bp) O2 < HCl < H2O2 (highest bp)
C) (lowest bp) HCl < H2O2 < O2 (highest bp)
D) (lowest bp) HCl < O2 < H2O2 (highest bp)
E) (lowest bp) O2 < H2O2 < HCl (highest bp)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
On the basis of intermolecular forces of attraction, rank the following three compounds in terms of increasing boiling point. CH3CH2CH2CH2CH3 (Pentane), CHCl3 (Chloroform) and H2O (Water)
A) (lowest bp) Pentane < Chloroform < Water (highest bp)
B) (lowest bp) Pentane < Water < Chloroform (highest bp)
C) (lowest bp) Water < Chloroform < Pentane (highest bp)
D) (lowest bp) Water < Pentane < Chloroform (highest bp)
E) (lowest bp) Chloroform < Water < Pentane (highest bp)
A) (lowest bp) Pentane < Chloroform < Water (highest bp)
B) (lowest bp) Pentane < Water < Chloroform (highest bp)
C) (lowest bp) Water < Chloroform < Pentane (highest bp)
D) (lowest bp) Water < Pentane < Chloroform (highest bp)
E) (lowest bp) Chloroform < Water < Pentane (highest bp)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
On the basis of intermolecular forces of attraction, rank the following three compounds in terms of increasing boiling point . NH3 (Ammonia), N2 (Nitrogen) and HCl (Hydrogen Chloride)
A) (lowest bp) Ammonia < Hydrogen chloride < Nitrogen (highest bp)
B) (lowest bp) Ammonia < Nitrogen < Hydrogen chloride (highest bp)
C) (lowest bp) Nitrogen < Hydrogen chloride < Ammonia (highest bp)
D) (lowest bp) Nitrogen < Ammonia < Hydrogen chloride (highest bp)
E) (lowest bp) Hydrogen chloride < Nitrogen < Ammonia (highest bp)
A) (lowest bp) Ammonia < Hydrogen chloride < Nitrogen (highest bp)
B) (lowest bp) Ammonia < Nitrogen < Hydrogen chloride (highest bp)
C) (lowest bp) Nitrogen < Hydrogen chloride < Ammonia (highest bp)
D) (lowest bp) Nitrogen < Ammonia < Hydrogen chloride (highest bp)
E) (lowest bp) Hydrogen chloride < Nitrogen < Ammonia (highest bp)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the hydrogen halides, HX (where X = F, Cl, Br, and I). Based upon a comparison between the intermolecular forces for each compound, which would be expected to have the highest boiling point ?
A) HF
B) HCl
C) HBr
D) HI
A) HF
B) HCl
C) HBr
D) HI

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
Given the three statements below, pick the best answer.
I. Dipole-dipole interactions should contribute to the intermolecular bonding forces of FBr.
II. London forces (instantaneous dipole) are the only intermolecular forces acting between H2 molecules.
III. London forces should be greater for I2 than Br2 because I2 is more polarizable.
A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all are correct
E) only III is correct
I. Dipole-dipole interactions should contribute to the intermolecular bonding forces of FBr.
II. London forces (instantaneous dipole) are the only intermolecular forces acting between H2 molecules.
III. London forces should be greater for I2 than Br2 because I2 is more polarizable.
A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all are correct
E) only III is correct

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
Given the three molecules listed below, which choice is best?
I. CH3OH
II. H2S
III. CH3CH3
A) Hydrogen bonding is the dominant intermolecular force only for I.
B) Hydrogen bonding is the dominant intermolecular force for I and II.
C) Hydrogen bonding is the dominant intermolecular force for I, II, and III.
D) Hydrogen bonding is not important for any of these molecules.
E) None of these choices is correct.
I. CH3OH
II. H2S
III. CH3CH3
A) Hydrogen bonding is the dominant intermolecular force only for I.
B) Hydrogen bonding is the dominant intermolecular force for I and II.
C) Hydrogen bonding is the dominant intermolecular force for I, II, and III.
D) Hydrogen bonding is not important for any of these molecules.
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
Three molecules are listed below. Will hydrogen bonding be important for any of them?
Pick the correct answer.
I. HBr
II. CH3OH
III. NH3
A) I and II will hydrogen bond, III will not
B) I and III will hydrogen bond, II will not
C) II and III will hydrogen bond, I will not
D) all three will hydrogen bond
E) only III will hydrogen bond
Pick the correct answer.
I. HBr
II. CH3OH
III. NH3
A) I and II will hydrogen bond, III will not
B) I and III will hydrogen bond, II will not
C) II and III will hydrogen bond, I will not
D) all three will hydrogen bond
E) only III will hydrogen bond

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
Pick the true statement below.
A) Dipole-dipole forces dominate the intermolecular forces for HBr.
B) The dominant intermolecular force for CH3NH2 is the dispersion force.
C) Hydrogen bonding is the most important intermolecular force for both CH3OH and CHCl3.
D) Hydrogen bonding is the most important intermolecular force for both HF and H2O.
E) All four of these are false.
A) Dipole-dipole forces dominate the intermolecular forces for HBr.
B) The dominant intermolecular force for CH3NH2 is the dispersion force.
C) Hydrogen bonding is the most important intermolecular force for both CH3OH and CHCl3.
D) Hydrogen bonding is the most important intermolecular force for both HF and H2O.
E) All four of these are false.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
Based upon intermolecular forces of attraction, which is the correct order for increasing boiling points among the following three molecules?
N2, H2, and NH3
A) (lowest bp) N2 < H2 < NH3 (highest bp)
B) (lowest bp) H2 < N2 < NH3 (highest bp)
C) (lowest bp) NH3 < H2 < N2 (highest bp)
D) (lowest bp) NH3 < N2 < H2 (highest bp)
E) (lowest bp) H2 < NH3 < N2 (highest bp)
N2, H2, and NH3
A) (lowest bp) N2 < H2 < NH3 (highest bp)
B) (lowest bp) H2 < N2 < NH3 (highest bp)
C) (lowest bp) NH3 < H2 < N2 (highest bp)
D) (lowest bp) NH3 < N2 < H2 (highest bp)
E) (lowest bp) H2 < NH3 < N2 (highest bp)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
Which molecule listed below would have its intermolecular forces dominated by dispersion forces?
A) N2
B) CH3OH
C) H2O
D) NH3
E) none of these
A) N2
B) CH3OH
C) H2O
D) NH3
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
Arrange the following elements in order of increasing boiling point. Argon, Helium, Neon
A) (lowest bp) Argon < Helium < Neon (highest bp)
B) (lowest bp) Argon < Neon < Helium (highest bp)
C) (lowest bp) Helium < Neon < Argon (highest bp)
D) (lowest bp) Helium < Argon < Neon (highest bp)
E) (lowest bp) Neon < Helium < Argon (highest bp)
A) (lowest bp) Argon < Helium < Neon (highest bp)
B) (lowest bp) Argon < Neon < Helium (highest bp)
C) (lowest bp) Helium < Neon < Argon (highest bp)
D) (lowest bp) Helium < Argon < Neon (highest bp)
E) (lowest bp) Neon < Helium < Argon (highest bp)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following forces between atoms or ions or molecules is the strongest?
A) Electrostatic force between ions
B) Ion-dipole force
C) Dipole-dipole force
D) London dispersion force
E) Hydrogen bonding
A) Electrostatic force between ions
B) Ion-dipole force
C) Dipole-dipole force
D) London dispersion force
E) Hydrogen bonding

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
Given the three statements below, pick the best answer.
I. Dipole-dipole forces are the main intermolecular force for I - Br.
II. O2 is a gas at room temperature because it is highly polarizable.
III. London forces dominate the intermolecular forces for Br2.
A) only I is true
B) only II is true
C) only III is true
D) II and III are true, I is false
E) I and III are true, II is false
I. Dipole-dipole forces are the main intermolecular force for I - Br.
II. O2 is a gas at room temperature because it is highly polarizable.
III. London forces dominate the intermolecular forces for Br2.
A) only I is true
B) only II is true
C) only III is true
D) II and III are true, I is false
E) I and III are true, II is false

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following molecules cannot form hydrogen bonds as a pure liquid?
A) H - F
B)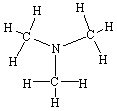
C)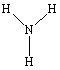
D)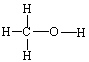
E) All of these can hydrogen bond
A) H - F
B)

C)

D)

E) All of these can hydrogen bond

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
On the basis of intermolecular forces of attraction, rank the following three compounds in terms of increasing boiling point . CH3CH2OH, CH3OCH3 and CH3CH2CH3
A) (lowest bp) CH3CH2OH < CH3OCH3 < CH3CH2CH3 (highest bp)
B) (lowest bp) CH3CH2OH < CH3CH2CH3 < CH3OCH3 (highest bp)
C) (lowest bp) CH3CH2CH3 < CH3OCH3 < CH3CH2OH (highest bp)
D) (lowest bp) CH3CH2CH3 < CH3CH2OH < CH3OCH3 (highest bp)
E) (lowest bp) CH3OCH3 < CH3CH2OH < CH3CH2CH3 (highest bp)
A) (lowest bp) CH3CH2OH < CH3OCH3 < CH3CH2CH3 (highest bp)
B) (lowest bp) CH3CH2OH < CH3CH2CH3 < CH3OCH3 (highest bp)
C) (lowest bp) CH3CH2CH3 < CH3OCH3 < CH3CH2OH (highest bp)
D) (lowest bp) CH3CH2CH3 < CH3CH2OH < CH3OCH3 (highest bp)
E) (lowest bp) CH3OCH3 < CH3CH2OH < CH3CH2CH3 (highest bp)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the molecules shown below does not have any dipole-dipole intermolecular forces?
A) PH3
B) BeH2
C) HBr
D) HCN
E) none of these
A) PH3
B) BeH2
C) HBr
D) HCN
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
What is the most important intermolecular force in solid H2O?
A) London dispersion
B) Dipole-dipole
C) Hydrogen bonding
D) Ionic bonding
E) Covalent bonding
A) London dispersion
B) Dipole-dipole
C) Hydrogen bonding
D) Ionic bonding
E) Covalent bonding

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following would have a boiling point lower than SiCl4?
A) LiCl
B) CCl4
C) SiBr4
D) GeCl4
E) none of these
A) LiCl
B) CCl4
C) SiBr4
D) GeCl4
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
Based upon an analysis of intermolecular forces of attraction among the compounds listed below, which substance would be expected to have the highest boiling point?
A) Br2
B) F2
C) N2
D) H2
E) I2
A) Br2
B) F2
C) N2
D) H2
E) I2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
The crystal forces in solid iodine are classified as:
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
The property of a liquid that causes small drops to be spherical is called:
A) enthalpy of vaporization.
B) viscosity.
C) surface tension.
D) vapor pressure.
E) none of these
A) enthalpy of vaporization.
B) viscosity.
C) surface tension.
D) vapor pressure.
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following properties of a liquid increase as the strength of intermolecular forces increases?
I. enthalpy of vaporization
II. boiling point
III. surface tension
A) I and II increase, III decreases
B) II and III increase, I decreases
C) I and III increase, II decreases
D) I, II, and III all increase
E) none of these are correct
I. enthalpy of vaporization
II. boiling point
III. surface tension
A) I and II increase, III decreases
B) II and III increase, I decreases
C) I and III increase, II decreases
D) I, II, and III all increase
E) none of these are correct

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
How many unit cells share an atom at the corner of a cubic unit cell?
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
The stacking of closest packing layers for a hexagonal closest packing is best described as:
A) ABABAB...
B) ABCABC...
C) ABBAABBA...
D) AABBAABB...
E) none of these
A) ABABAB...
B) ABCABC...
C) ABBAABBA...
D) AABBAABB...
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
Which crystal packing structure listed below provides an "ABA" closest packing arrangement for the different layers of metal spheres?
A) Hexagonal Close pack
B) Face Centered Cubic
C) Body Centered Cubic
D) Primitive Cubic
E) None of these
A) Hexagonal Close pack
B) Face Centered Cubic
C) Body Centered Cubic
D) Primitive Cubic
E) None of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
Which crystal packing structure listed below provides an "ABC" closest packing arrangement for the different layers of metal spheres?
A) Hexagonal Close pack
B) Face Centered Cubic
C) Body Centered Cubic
D) Primitive Cubic
E) None of these
A) Hexagonal Close pack
B) Face Centered Cubic
C) Body Centered Cubic
D) Primitive Cubic
E) None of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
The property of a liquid that measures its resistance to flowing is:
A) enthalpy of vaporization.
B) viscosity.
C) surface tension.
D) vapor pressure.
E) none of these
A) enthalpy of vaporization.
B) viscosity.
C) surface tension.
D) vapor pressure.
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
What is the name of the cubic unit cell shown below?
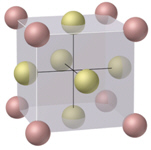
A) Primitive Cubic
B) Body Centered Cubic
C) Face Centered Cubic
D) Edge Centered Cubic
E) None of these

A) Primitive Cubic
B) Body Centered Cubic
C) Face Centered Cubic
D) Edge Centered Cubic
E) None of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
A lithium fluoride crystal is classified as:
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
What type of crystal would CO2 form upon solidification?
A) Molecular
B) Ionic
C) Covalent network
D) Metallic
E) Pyramidal
A) Molecular
B) Ionic
C) Covalent network
D) Metallic
E) Pyramidal

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
An atom sitting in the center position in a body-centered cubic cell is shared among how many cubes?
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
The basic repeating three-dimensional pattern of a solid is known as the:
A) Bragg cell
B) closest packing
C) covalent network
D) unit cell
E) none of these
A) Bragg cell
B) closest packing
C) covalent network
D) unit cell
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
In a closest packing array of atoms, how many nearest neighbors does each atom have?
A) 4
B) 6
C) 8
D) 12
E) none of these
A) 4
B) 6
C) 8
D) 12
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
A diamond crystal is classified as:
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
Iron crystallizes in a body-centered cubic array of atoms. What is the number of atoms per unit cell for this metal?
A) 1
B) 2
C) 9
D) 4
E) 12
A) 1
B) 2
C) 9
D) 4
E) 12

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
Cohesive forces of attraction are intermolecular attractions:
A) between molecules of two or more different substances.
B) that are covalent bonds.
C) between molecules of the same substance.
D) that occur between polar molecules only.
E) none of these
A) between molecules of two or more different substances.
B) that are covalent bonds.
C) between molecules of the same substance.
D) that occur between polar molecules only.
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
The term that best explains why water rises up a thin glass tube is:
A) capillary action.
B) heat of vaporization.
C) viscosity.
D) surface tension.
E) none of these
A) capillary action.
B) heat of vaporization.
C) viscosity.
D) surface tension.
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
In a cubic array of atoms, how many unit cells share an atom in the center of a face?
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
A copper crystal is classified as:
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these
A) ionic
B) molecular
C) covalent network
D) metallic
E) none of these

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


