Exam 11: Liquids and Solids
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
What is the name of the cubic unit cell shown below?
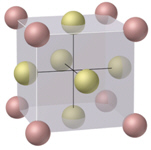
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
C
A slush of liquid water and ice crystals is at equilibrium at 0 ° C. If a small amount of heat is removed so that more ice is formed, the system will experience:
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
E
Consider the hydrogen halides, HX (where X = F, Cl, Br, and I). Based upon a comparison between the intermolecular forces for each compound, which would be expected to have the highest boiling point ?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
Exhibit 11-1 The heating curve below is needed for the following question(s). 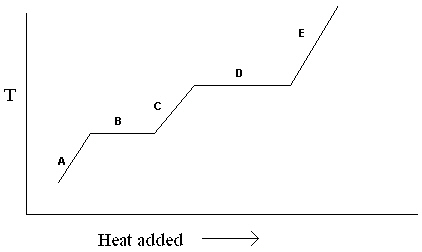 Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment that corresponds to the heat of fusion?
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment that corresponds to the heat of fusion?
(Multiple Choice)
5.0/5  (36)
(36)
Exhibit 11-1 The heating curve below is needed for the following question(s). 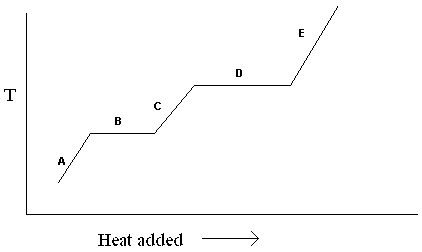 Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where only liquid is present?
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where only liquid is present?
(Multiple Choice)
5.0/5  (40)
(40)
What intermolecular force(s) of attraction is(are) present between two molecules of CH2F2?
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
(Multiple Choice)
4.8/5  (31)
(31)
Which of the molecules shown below does not have any dipole-dipole intermolecular forces?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following molecules can form hydrogen bonds ?
I. CH3NH2
II. CH3OH
III. CH3F
(Multiple Choice)
4.8/5  (24)
(24)
What intermolecular force(s) of attraction is(are) present between two molecules of ethanol shown below?
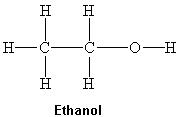 I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
(Multiple Choice)
4.8/5  (34)
(34)
Arrange the following molecules in order of increasing boiling point. H2O2, HCl and O2
(Multiple Choice)
4.8/5  (40)
(40)
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 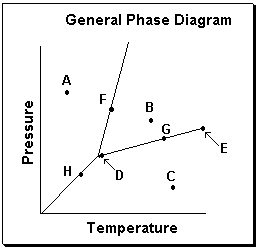 -Refer to Exhibit 11-3. What phase transition would occur upon moving from point B to point C in the phase diagram above?
-Refer to Exhibit 11-3. What phase transition would occur upon moving from point B to point C in the phase diagram above?
(Multiple Choice)
4.8/5  (38)
(38)
The stacking of closest packing layers for a hexagonal closest packing is best described as:
(Multiple Choice)
4.8/5  (38)
(38)
Gold crystallizes in a face-centered cubic array of atoms. How many atoms are present per unit cell for the metal?
(Multiple Choice)
5.0/5  (25)
(25)
Which of the following molecules cannot form hydrogen bonds as a pure liquid?
(Multiple Choice)
4.7/5  (44)
(44)
The temperature at which solid is in equilibrium with its vapor is called
(Multiple Choice)
4.9/5  (43)
(43)
Cohesive forces of attraction are intermolecular attractions:
(Multiple Choice)
4.8/5  (27)
(27)
The edge of the face-centered cubic unit cell of a metal is 376 pm. What is the radius of the metal atom?
(Multiple Choice)
4.8/5  (36)
(36)
What is the transition from the gaseous phase to the solid phase?
(Multiple Choice)
4.9/5  (39)
(39)
Which phase transition listed below would represent the transition from the solid state to gaseous vapor?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 1 - 20 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)