Deck 10: Solids, Liquids, and Phase Transitions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 10: Solids, Liquids, and Phase Transitions
1
The density of a gas is typically
A) 1000 times smaller than a liquid.
B) 10 times smaller than a liquid.
C) approximately the same as a liquid.
D) 10 times greater than a liquid.
E) 1000 times greater than a liquid.
A) 1000 times smaller than a liquid.
B) 10 times smaller than a liquid.
C) approximately the same as a liquid.
D) 10 times greater than a liquid.
E) 1000 times greater than a liquid.
A
2
The potential energy of a molecule at the surface of a liquid is
A) higher than that of a molecule in the bulk.
B) lower than that of a molecule in the bulk.
C) exactly the same as that of a molecule in the bulk.
D) higher or lower depending on the liquid.
E) higher or lower depending on the pressure.
A) higher than that of a molecule in the bulk.
B) lower than that of a molecule in the bulk.
C) exactly the same as that of a molecule in the bulk.
D) higher or lower depending on the liquid.
E) higher or lower depending on the pressure.
A
3
The thermal expansion coefficient of most liquids is
A) larger than that for most solids.
B) larger than that for most gases.
C) smaller than that for most solids.
D) smaller than that for most gases.
E) b & c
F) a & d
A) larger than that for most solids.
B) larger than that for most gases.
C) smaller than that for most solids.
D) smaller than that for most gases.
E) b & c
F) a & d
F
4
Which has the highest vapor pressure at room temperature?
A) methanol
B) ethanol
C) propanol
D) butanol
E) there is no way to predict
A) methanol
B) ethanol
C) propanol
D) butanol
E) there is no way to predict

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Which do you predict will have the largest intermolecular attraction F2, Cl2, Br2, I2?
A) F2
B) Cl2
C) Br2
D) I2
E) they will all be the same
A) F2
B) Cl2
C) Br2
D) I2
E) they will all be the same

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
What types of intermolecular forces would you expect to find in CHCl3?
A) dispersion
B) dipole-dipole
C) hydrogen bonding
D) a & b
E) all of the above
A) dispersion
B) dipole-dipole
C) hydrogen bonding
D) a & b
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
Generally if a liquid has stronger intermolecular attractions it will have
A) a higher viscosity.
B) a higher vapor pressure.
C) a higher boiling point.
D) a & b
E) b & c
F) a & c
G) all of the above
A) a higher viscosity.
B) a higher vapor pressure.
C) a higher boiling point.
D) a & b
E) b & c
F) a & c
G) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Rank the following in order of increasing boiling point.
CHCl3, Ne, H2O, MgO
A) CHCl3, Ne, H2O, MgO
B) MgO, Ne, H2O, CHCl3
C) Ne, H2O, MgO, CHCl3
D) Ne, CHCl3, H2O, MgO
E) MgO, CHCl3, H2O, Ne
CHCl3, Ne, H2O, MgO
A) CHCl3, Ne, H2O, MgO
B) MgO, Ne, H2O, CHCl3
C) Ne, H2O, MgO, CHCl3
D) Ne, CHCl3, H2O, MgO
E) MgO, CHCl3, H2O, Ne

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
The boiling point of CCl4 is slightly higher than that of CHCl3. The boiling point of CHF3 is significantly higher than CF4. Why is this?
A) The dipole of CHF3 is larger than that of CHCl3.
B) The dipole of CHF3 is smaller than that of CHCl3.
C) The dispersion forces are larger in CF4 than CCl4.
D) The dispersion forces are larger in CF4 than CCl4.
E) b & c
F) a & d
A) The dipole of CHF3 is larger than that of CHCl3.
B) The dipole of CHF3 is smaller than that of CHCl3.
C) The dispersion forces are larger in CF4 than CCl4.
D) The dispersion forces are larger in CF4 than CCl4.
E) b & c
F) a & d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
The vapor pressure of methanol (CH3OH) at 25°C is 93.3 Torr. If 10 g of methanol is placed into an evacuated 10 L container at a constant temperature of 25°C, what is the final pressure in the container?
A) 58.1 Torr
B) 93.3 Torr
C) 376 Torr
D) 1 atm
E) none of the above
A) 58.1 Torr
B) 93.3 Torr
C) 376 Torr
D) 1 atm
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
The vapor pressure of methanol (CH3OH) at 25°C is 93.3 Torr. If 1 g of methanol is placed into an evacuated 10 L container at a constant temperature of 25°C, what is the final pressure in the container?
A) 58.1 Torr
B) 93.3 Torr
C) 376 Torr
D) 1 atm
E) none of the above
A) 58.1 Torr
B) 93.3 Torr
C) 376 Torr
D) 1 atm
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
At 20°C the vapor pressure of dry ice is 56.5 atm. If 100 g of dry ice is place in an evacuated 0.5 L chamber at a constant temperature of 20°C, how many grams of solid will sublime?
A) 0.0872 g
B) 10.1 g
C) 28.1 g
D) 51.7 g
E) 100 g
A) 0.0872 g
B) 10.1 g
C) 28.1 g
D) 51.7 g
E) 100 g

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
At 20°C water has a vapor pressure of 0.0231 atm. If you bubble air through a container of water and collect 1 L of it at a total pressure of 1 atm, how many grams of water are in your sample of air (you can assume it is saturated)?
A) 9.6×10-4
B) 3.0×10-4
C) 3.0×10-3
D) 1.1×10-2
E) 1.7×10-2
A) 9.6×10-4
B) 3.0×10-4
C) 3.0×10-3
D) 1.1×10-2
E) 1.7×10-2

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
A liquid kinetically trapped below its freezing temperature is called a
A) glassy liquid.
B) condensed liquid.
C) fluid liquid.
D) supercooled liquid.
E) frozen liquid.
A) glassy liquid.
B) condensed liquid.
C) fluid liquid.
D) supercooled liquid.
E) frozen liquid.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is true for a liquid at its equilibrium vapor pressure?
A) the rate of evaporation exceeds that of condensation.
B) the rate of condensation exceeds that of evaporation.
C) the rate of condensation and evaporation are the same.
D) the system is neither evaporating nor condensing.
E) the system is at the critical point.
A) the rate of evaporation exceeds that of condensation.
B) the rate of condensation exceeds that of evaporation.
C) the rate of condensation and evaporation are the same.
D) the system is neither evaporating nor condensing.
E) the system is at the critical point.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
A supercritical fluid has a density that
A) is lower than a liquid.
B) is lower than a gas.
C) is higher than a liquid.
D) is higher than a gas.
E) can be varied from gas-like to liquid-like.
A) is lower than a liquid.
B) is lower than a gas.
C) is higher than a liquid.
D) is higher than a gas.
E) can be varied from gas-like to liquid-like.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
At the triple point
A) solid, liquid, and gas are all in equilibrium.
B) the vapor pressure of the solid is equal to the vapor pressure of the liquid.
C) the rate of sublimation is equal to the rate of evaporation.
D) a & b
E) all of the above
A) solid, liquid, and gas are all in equilibrium.
B) the vapor pressure of the solid is equal to the vapor pressure of the liquid.
C) the rate of sublimation is equal to the rate of evaporation.
D) a & b
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
For the following phase diagram the area labeled IV is 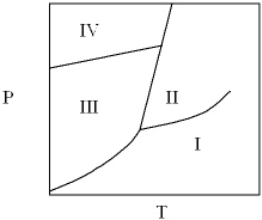
A) gas
B) liquid
C) solid
D) super critical fluid
E) supercooled liquid

A) gas
B) liquid
C) solid
D) super critical fluid
E) supercooled liquid

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
The process of a solid converting directly to a gas is called
A) evaporation
B) condensation
C) deposition
D) sublimation
E) transformation
A) evaporation
B) condensation
C) deposition
D) sublimation
E) transformation

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
Given the phase diagram below, at 200K and a pressure of 150 atm. What phase(s) is (are) present? 
A) gas
B) liquid
C) solid
D) liquid and solid
E) gas and liquid

A) gas
B) liquid
C) solid
D) liquid and solid
E) gas and liquid

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
The triple point of a compound is at 38.2°C and a pressure of 0.75 atm. A gaseous sample of the substance is held at a constant pressure of 0.79 atm. Initially the sample is at 50°C and is slowly cooled until it is -25°C. What if any phase transitions occur?
A) condensation
B) deposition
C) condensation followed by freezing
D) simultaneous condensation and freezing
E) none of the above
A) condensation
B) deposition
C) condensation followed by freezing
D) simultaneous condensation and freezing
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
For a given substance, the vapor pressure of a liquid can never be
A) equal to the pressure at the triple point.
B) equal to the critical temperature.
C) lower than the vapor pressure of the solid.
D) higher than the vapor pressure of the solid.
E) greater than 1 atm.
A) equal to the pressure at the triple point.
B) equal to the critical temperature.
C) lower than the vapor pressure of the solid.
D) higher than the vapor pressure of the solid.
E) greater than 1 atm.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Which do you think will have the highest surface tension?
A) water
B) liquid N2
C) CCl4
D) CHCl3
E) molten NaCl
A) water
B) liquid N2
C) CCl4
D) CHCl3
E) molten NaCl

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Which do you think has a higher boiling point, n-butanol or tert-butanol? 
A) n-butanol because it forms stronger hydrogen bonds.
B) tert-butanol because it forms stronger hydrogen bonds.
C) n-butanol because the linear molecule allows for greater dispersion forces.
D) tert-butanol because the tetrahedral molecule allows for greater dispersion forces.
E) they will be exactly the same.

A) n-butanol because it forms stronger hydrogen bonds.
B) tert-butanol because it forms stronger hydrogen bonds.
C) n-butanol because the linear molecule allows for greater dispersion forces.
D) tert-butanol because the tetrahedral molecule allows for greater dispersion forces.
E) they will be exactly the same.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
In the phase diagram of water increasing pressure leads to lower melting temperatures.
A) this is true for all compounds
B) this is true for all polar compounds
C) this is true for all hydrogen bonding compounds
D) this is true for compounds with solid phases that are less dense than their liquid phases
E) this is true only for H2O, HF, and NH3
A) this is true for all compounds
B) this is true for all polar compounds
C) this is true for all hydrogen bonding compounds
D) this is true for compounds with solid phases that are less dense than their liquid phases
E) this is true only for H2O, HF, and NH3

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Dipole-dipole forces are
A) larger than ion-ion forces.
B) about the same as all ion-ion forces.
C) much smaller than ion-ion forces.
D) between the force for °1 ions and °2 ions.
E) about a factor of two smaller the attraction of °1 ions.
A) larger than ion-ion forces.
B) about the same as all ion-ion forces.
C) much smaller than ion-ion forces.
D) between the force for °1 ions and °2 ions.
E) about a factor of two smaller the attraction of °1 ions.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
While many solids are crystalline with a well ordered structure of molecules, glasses are amorphous solids in which the structure of the molecules is random. How is a glass different from a liquid?
A) The glass will have a lower diffusion coefficient.
B) The glass will be rigid.
C) The glass will be more compressible.
D) a & b
E) all of the above
A) The glass will have a lower diffusion coefficient.
B) The glass will be rigid.
C) The glass will be more compressible.
D) a & b
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Arrange the follow in order of increasing boiling point: H2O, H2S, H2Se, H2Te
A) H2O, H2S, H2Se, H2Te
B) H2Te, H2Se, H2S, H2O
C) H2O, H2Te, H2Se, H2S
D) H2S, H2Se, H2Te, H2O
E) H2S, H2Se, H2O, H2Te
A) H2O, H2S, H2Se, H2Te
B) H2Te, H2Se, H2S, H2O
C) H2O, H2Te, H2Se, H2S
D) H2S, H2Se, H2Te, H2O
E) H2S, H2Se, H2O, H2Te

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
The critical temperature for methanol is 512.6 K and the critical pressure is 81 bar. If a sample starts at a temperature of 590 K and a pressure of 45.3 bar and is cooled at constant pressure to a temperature of 512.6 K, what if any, phase transition are observed?
A) condensation
B) evaporation
C) condensation followed by evaporation
D) there are no phase transitions
A) condensation
B) evaporation
C) condensation followed by evaporation
D) there are no phase transitions

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
The critical temperature for cyclohexane is 553.5 K and the critical pressure is 40.7 bar. A sample of cyclohexane at a temperature of 532 K and a pressure of 40.7 is
A) a gas
B) a liquid
C) a super critical fluid
D) a super heated fluid
E) a super heated vapor
A) a gas
B) a liquid
C) a super critical fluid
D) a super heated fluid
E) a super heated vapor

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
The process of a gas converting directly to a solid is
A) evaporation
B) condensation
C) sublimation
D) deposition
E) freezing
A) evaporation
B) condensation
C) sublimation
D) deposition
E) freezing

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is the most polarizable?
A) He
B) Xe
C) Ar
D) Kr
E) Ne
A) He
B) Xe
C) Ar
D) Kr
E) Ne

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Based on intermolecular forces, which organic compound should have the highest boiling point?
A) Propane
B) Dimethyl ketone (acetone)
C) Ethanol
D) Propanol
E) Hexane
A) Propane
B) Dimethyl ketone (acetone)
C) Ethanol
D) Propanol
E) Hexane

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


