Exam 10: Solids, Liquids, and Phase Transitions
Exam 1: The Atom in Modern Chemistry33 Questions
Exam 2: Chemical Formulas, Equations, and Reaction Yields33 Questions
Exam 3: Chemical Bonding: the Classical Description33 Questions
Exam 4: Introduction to Quantum Mechanics33 Questions
Exam 5: Quantum Mechanics and Atomic Structure33 Questions
Exam 6: Quantum Mechanics and Molecular Structure33 Questions
Exam 7: Bonding in Organic Molecules33 Questions
Exam 8: Binding in Transition Metal Compounds and Coordination Complexes33 Questions
Exam 9: The Gaseous State33 Questions
Exam 10: Solids, Liquids, and Phase Transitions33 Questions
Exam 11: Solutions33 Questions
Exam 12: Thermodynamic Processes and Thermochemistry33 Questions
Exam 13: Spontaneous Processes and Thermodynamic Equilibrium33 Questions
Exam 14: Chemical Equilibrium34 Questions
Exam 15: Acid-Base Equilibria33 Questions
Exam 16: Solubility and Precipitation Equilibria33 Questions
Exam 17: Electrochemistry33 Questions
Exam 18: Chemical Kinetics33 Questions
Exam 19: Nuclear Chemistry33 Questions
Exam 20: Molecular Spectroscopy and Photochemistry33 Questions
Exam 21: Structure and Bonding in Solids33 Questions
Exam 22: Inorganic Materials33 Questions
Exam 23: Polymeric Materials and Soft Condensed Matter33 Questions
Select questions type
For the following phase diagram the area labeled IV is 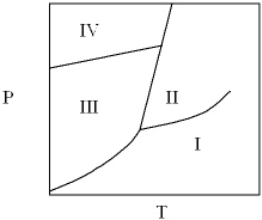
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
Which of the following is the most polarizable?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B
The process of a gas converting directly to a solid is
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
D
While many solids are crystalline with a well ordered structure of molecules, glasses are amorphous solids in which the structure of the molecules is random. How is a glass different from a liquid?
(Multiple Choice)
4.7/5  (41)
(41)
Given the phase diagram below, at 200K and a pressure of 150 atm. What phase(s) is (are) present? 
(Multiple Choice)
4.7/5  (37)
(37)
The vapor pressure of methanol (CH3OH) at 25°C is 93.3 Torr. If 1 g of methanol is placed into an evacuated 10 L container at a constant temperature of 25°C, what is the final pressure in the container?
(Multiple Choice)
4.8/5  (39)
(39)
For a given substance, the vapor pressure of a liquid can never be
(Multiple Choice)
4.9/5  (41)
(41)
The potential energy of a molecule at the surface of a liquid is
(Multiple Choice)
4.9/5  (34)
(34)
The critical temperature for cyclohexane is 553.5 K and the critical pressure is 40.7 bar. A sample of cyclohexane at a temperature of 532 K and a pressure of 40.7 is
(Multiple Choice)
4.9/5  (28)
(28)
The critical temperature for methanol is 512.6 K and the critical pressure is 81 bar. If a sample starts at a temperature of 590 K and a pressure of 45.3 bar and is cooled at constant pressure to a temperature of 512.6 K, what if any, phase transition are observed?
(Multiple Choice)
4.9/5  (37)
(37)
Rank the following in order of increasing boiling point.
CHCl3, Ne, H2O, MgO
(Multiple Choice)
4.9/5  (43)
(43)
Based on intermolecular forces, which organic compound should have the highest boiling point?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following is true for a liquid at its equilibrium vapor pressure?
(Multiple Choice)
5.0/5  (38)
(38)
The vapor pressure of methanol (CH3OH) at 25°C is 93.3 Torr. If 10 g of methanol is placed into an evacuated 10 L container at a constant temperature of 25°C, what is the final pressure in the container?
(Multiple Choice)
4.8/5  (34)
(34)
At 20°C the vapor pressure of dry ice is 56.5 atm. If 100 g of dry ice is place in an evacuated 0.5 L chamber at a constant temperature of 20°C, how many grams of solid will sublime?
(Multiple Choice)
4.8/5  (37)
(37)
The boiling point of CCl4 is slightly higher than that of CHCl3. The boiling point of CHF3 is significantly higher than CF4. Why is this?
(Multiple Choice)
4.8/5  (31)
(31)
A liquid kinetically trapped below its freezing temperature is called a
(Multiple Choice)
4.7/5  (39)
(39)
Arrange the follow in order of increasing boiling point: H2O, H2S, H2Se, H2Te
(Multiple Choice)
4.9/5  (39)
(39)
Showing 1 - 20 of 33
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)