Deck 2: An Introduction to the Chemical Basis of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 2: An Introduction to the Chemical Basis of Life
1
Which of the following elements is a bacterial micronutrient?
A) carbon
B) chlorine
C) hydrogen
D) nitrogen
A) carbon
B) chlorine
C) hydrogen
D) nitrogen
B
2
The chemical symbol for ________ is ________. (Select all that apply)
A) carbon; Ca
B) oxygen; O
C) potassium; K
D) magnesium; Mn
A) carbon; Ca
B) oxygen; O
C) potassium; K
D) magnesium; Mn
B,C
3
Elements with similar chemical properties are organized in the same ________ of the periodic table.
A) row
B) quadrant
C) column
D) color block
A) row
B) quadrant
C) column
D) color block
C
4
The atomic nucleus of an atom is composed of ________.
A) positively charged protons
B) positively protons and electrically neutral neutrons
C) electrically neutral neutrons and negatively charged electrons
D) electrically neutral protons
A) positively charged protons
B) positively protons and electrically neutral neutrons
C) electrically neutral neutrons and negatively charged electrons
D) electrically neutral protons

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
Each proton and neutron have a mass of one atomic mass unit (amu). Using this information from the periodic table, determine the mass of sodium (Na).

A) 11 amu
B) 12 amu
C) 23 amu
D) 34 amu

A) 11 amu
B) 12 amu
C) 23 amu
D) 34 amu

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Isotopes of an element differ from each other by the ________.
A) number of neutrons they possess
B) number of valence shell electrons
C) arrangement of their electrons in orbitals
D) arrangement of their electrons in the atomic nucleus
A) number of neutrons they possess
B) number of valence shell electrons
C) arrangement of their electrons in orbitals
D) arrangement of their electrons in the atomic nucleus

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
Radioisotopes of an element ________. (Select all that apply)
A) are highly reactive and preferentially participate in chemical reactions
B) emit energy
C) can be used in diagnostic procedures such as indium scans
D) have additional electrons in their valence shell
A) are highly reactive and preferentially participate in chemical reactions
B) emit energy
C) can be used in diagnostic procedures such as indium scans
D) have additional electrons in their valence shell

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
Orbitals ________. (Select all that apply)
A) represent the volume of space where an atom's electrons are most likely to be found
B) are associated with different energy shells
C) contain a maximum of 8 electrons
D) may be spherical or pear-shaped
A) represent the volume of space where an atom's electrons are most likely to be found
B) are associated with different energy shells
C) contain a maximum of 8 electrons
D) may be spherical or pear-shaped

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
Valence shell electrons ________. (Select all that apply)
A) are located exclusively in the s orbitals
B) participate in chemical reactions
C) have the highest associated energy
D) can be shared with the valence shell of an adjacent atom
A) are located exclusively in the s orbitals
B) participate in chemical reactions
C) have the highest associated energy
D) can be shared with the valence shell of an adjacent atom

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
When an atom loses a valence shell electron, it ________. (Select all that apply)
A) becomes a cation
B) participates in polar covalent bonds
C) can become part of a polar molecule
D) can form an ionic bond with an anion
A) becomes a cation
B) participates in polar covalent bonds
C) can become part of a polar molecule
D) can form an ionic bond with an anion

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
When two adjacent atoms of similar electronegativity share a pair of electrons, ________. (Select all that apply)
A) it promotes the formation of hydrogen bonds with surrounding atoms
B) a strong polar covalent bond is formed
C) a strong nonpolar covalent bond is formed
D) van der Waals forces are generated
A) it promotes the formation of hydrogen bonds with surrounding atoms
B) a strong polar covalent bond is formed
C) a strong nonpolar covalent bond is formed
D) van der Waals forces are generated

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
When two adjacent atoms unequally share a pair of electrons, ________. (Select all that apply)
A) the resulting bond is highly unstable
B) a strong polar covalent bond is formed
C) a strong nonpolar covalent bond is formed
D) a polar molecule is formed
A) the resulting bond is highly unstable
B) a strong polar covalent bond is formed
C) a strong nonpolar covalent bond is formed
D) a polar molecule is formed

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
Two adjacent nitrogen atoms share 3 pairs of electrons resulting in the formation of ________.
A) a nonpolar covalent bond
B) a triple nonpolar covalent bond
C) a combination of polar and nonpolar covalent bonds
D) a triple polar covalent bond
A) a nonpolar covalent bond
B) a triple nonpolar covalent bond
C) a combination of polar and nonpolar covalent bonds
D) a triple polar covalent bond

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
When a pair of valence shell electrons are shared by two atoms with significantly different electronegativity values, ________.
A) a polar covalent bond forms
B) the electron pair of the bond is positioned closer to the atom with the lower electronegativity
C) an ionic bond forms
D) the electronegativity values of the two atoms equalize
A) a polar covalent bond forms
B) the electron pair of the bond is positioned closer to the atom with the lower electronegativity
C) an ionic bond forms
D) the electronegativity values of the two atoms equalize

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
Weak electrostatic attraction between a hydrogen atom participating in a polar covalent bond and a slightly negative region of a molecule results in the formation of a(n) ________.
A) ionic bond
B) double covalent bond
C) van der Waals interaction
D) hydrogen bond
A) ionic bond
B) double covalent bond
C) van der Waals interaction
D) hydrogen bond

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
In the chemical equation below, glucose is a(n) ________.
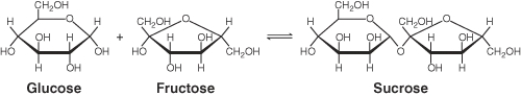
A) reactant
B) product
C) intermediate complex
D) equilabrator

A) reactant
B) product
C) intermediate complex
D) equilabrator

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
Chemical equilibrium describes ________.
A) a reaction that has run to completion
B) a reaction that has converted all reactant into product
C) a reaction that involves only one reactant and one product
D) a situation where the rate of the forward reaction is exactly equal to the rate of the reverse reaction
A) a reaction that has run to completion
B) a reaction that has converted all reactant into product
C) a reaction that involves only one reactant and one product
D) a situation where the rate of the forward reaction is exactly equal to the rate of the reverse reaction

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
Hydrogen bonds between water molecules in solid form ________. (Select all that apply)
A) make it less dense than its liquid form
B) allow it to serve as a layer of surface insulation to provide a thermally stable aquatic habitat in the winter
C) are very stable and space the molecules closer together
D) can convert to covalent bonds when ice melts
A) make it less dense than its liquid form
B) allow it to serve as a layer of surface insulation to provide a thermally stable aquatic habitat in the winter
C) are very stable and space the molecules closer together
D) can convert to covalent bonds when ice melts

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
Because of its extreme hydrogen bonding, water has a high specific heat so it ________. (Select all that apply)
A) heats up slowly
B) cools down slowly
C) maintains a thermally stable aquatic environment
D) requires the addition of a lot energy to break all of these weak intermolecular bonds
A) heats up slowly
B) cools down slowly
C) maintains a thermally stable aquatic environment
D) requires the addition of a lot energy to break all of these weak intermolecular bonds

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
Evaporation of sweat ________. (Select all that apply)
A) leads to cooling that is essential for maintaining a constant body temperature
B) is due to water's high specific heat
C) elevates the pH level of the skin
D) leaves elevated salt levels on the skin which serve to inhibit pathogen growth
A) leads to cooling that is essential for maintaining a constant body temperature
B) is due to water's high specific heat
C) elevates the pH level of the skin
D) leaves elevated salt levels on the skin which serve to inhibit pathogen growth

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
What unique feature of water is highlighted in this photo?

A) Water's ability to serve as an excellent solvent
B) Water's high specific heat
C) Water's high heat of vaporization
D) Water's cohesive nature

A) Water's ability to serve as an excellent solvent
B) Water's high specific heat
C) Water's high heat of vaporization
D) Water's cohesive nature

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is FALSE?
A) Water is a polar molecule
B) Water exists in nature in all three physical states
C) Hydrogen bonds tend to form between water molecules
D) Solid water is (ice) is denser than liquid water
A) Water is a polar molecule
B) Water exists in nature in all three physical states
C) Hydrogen bonds tend to form between water molecules
D) Solid water is (ice) is denser than liquid water

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
A ________ is the substance used to dissolve materials.
A) solute
B) solvent
C) solution
D) hydration shell
A) solute
B) solvent
C) solution
D) hydration shell

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
An aqueous solution ________. (Select all that apply)
A) uses water as a solvent
B) dissolves hydrophobic solutes
C) forms hydration shells around the solutes
D) is always characterized by a neutral pH
A) uses water as a solvent
B) dissolves hydrophobic solutes
C) forms hydration shells around the solutes
D) is always characterized by a neutral pH

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
The molecular weight of glucose is 181.18 g/mol. To make 250 ml of a 0.25 M glucose solution, you would dissolve ________ g of glucose in 250 ml of water.
A) 11.32
B) 18.11
C) 45.26
D) 181.18
A) 11.32
B) 18.11
C) 45.26
D) 181.18

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
Acids ________. (Select all that apply)
A) accept hydrogen ions from a solution
B) increase the pH when a buffer is added
C) have a pH value below 7
D) turn phenol red fuchsia
A) accept hydrogen ions from a solution
B) increase the pH when a buffer is added
C) have a pH value below 7
D) turn phenol red fuchsia

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
Acid rain is a serious environmental problem. A sample of rainwater collected in the Adirondack Mountains had an H+ concentration of 10-4 mol/L. The pH of this sample was ________.
A) 0.0001
B) -4
C) 4
D) 10,000
A) 0.0001
B) -4
C) 4
D) 10,000

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
If the pH of a solution is increased from pH 5 to pH 7, it means that the concentration of H+ is ________ than it was at pH 5.
A) 2 times lower
B) 10 times greater
C) 100 times greater
D) 100 times lower
A) 2 times lower
B) 10 times greater
C) 100 times greater
D) 100 times lower

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
To minimize the pH change when an acid or base is added to a solution, a ________ is needed.
A) ketone
B) pH indicator
C) isotope
D) buffer
A) ketone
B) pH indicator
C) isotope
D) buffer

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following represent ways to increase the molecular diversity of organic molecules? (Select all that apply)
A) varying the length of the carbon skeleton
B) the presence of multiple bonds in the carbon skeleton
C) branching of the carbon skeleton
D) substitution of functional groups for a hydrogen
A) varying the length of the carbon skeleton
B) the presence of multiple bonds in the carbon skeleton
C) branching of the carbon skeleton
D) substitution of functional groups for a hydrogen

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
Which functional group acts as an acid by donating a hydrogen ion to the solution?
A) Ketone
B) Methyl
C) Carboxyl
D) Hydroxyl
A) Ketone
B) Methyl
C) Carboxyl
D) Hydroxyl

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
Identify this functional group.
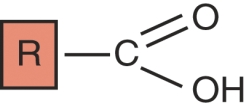
A) aldehyde
B) thiol
C) carboxyl
D) hydroxyl

A) aldehyde
B) thiol
C) carboxyl
D) hydroxyl

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
How many covalent bonds can be formed by one carbon atom?
A) 1
B) 2
C) 4
D) 8
A) 1
B) 2
C) 4
D) 8

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
Glucose and fructose represent ________ isomers. (Select all that apply)
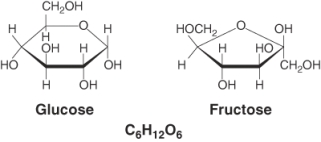
A) structural
B) optical
C) enantiomer
D) radioactive

A) structural
B) optical
C) enantiomer
D) radioactive

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
The figure below illustrates ________ isomers. (Select all that apply)
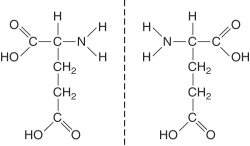
A) structural
B) optical
C) enantiomer
D) radioactive

A) structural
B) optical
C) enantiomer
D) radioactive

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
What is the chemical mechanism by which cells degrade polymers into monomers?
A) Ionic monomer bonding
B) Dehydration synthesis
C) Hydrolysis
D) Polymerization
A) Ionic monomer bonding
B) Dehydration synthesis
C) Hydrolysis
D) Polymerization

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
What process does a cell use to recycle monomers?
A) Ionic monomer bonding
B) Dehydration synthesis
C) Hydrolysis
D) Polymerization
A) Ionic monomer bonding
B) Dehydration synthesis
C) Hydrolysis
D) Polymerization

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck


