Exam 2: An Introduction to the Chemical Basis of Life
Exam 1: Microbial World61 Questions
Exam 2: An Introduction to the Chemical Basis of Life37 Questions
Exam 3: The Biochemistry of Macromolecules59 Questions
Exam 4: Microscopy53 Questions
Exam 5: Prokaryote Organisms92 Questions
Exam 6: Eukaryotic Cells50 Questions
Exam 7: Eukaryotic Organisms50 Questions
Exam 8: Viruses and Infectious Particles72 Questions
Exam 9: Metabolism60 Questions
Exam 10: Microbial Genetics and Genetic Engineering99 Questions
Exam 11: Microbial Growth and Control108 Questions
Exam 12: Antimicrobial Agents145 Questions
Exam 13: Innate Immunity63 Questions
Exam 14: Adaptive Immunity70 Questions
Exam 15: Vaccination, Immunoassays, and Immune Disorders74 Questions
Exam 16: Microbial Pathogenesis95 Questions
Exam 17: Epidemiology and Infection Control74 Questions
Exam 18: Diseases of the Respiratory System50 Questions
Exam 19: Diseases of the Skin and Eyes49 Questions
Exam 20: Diseases of the Gastrointestinal System50 Questions
Exam 21: Diseases of the Urogenital System50 Questions
Exam 22: Diseases of the Nervous System49 Questions
Exam 23: Diseases of the Cardiovascular and Lymphatic Systems48 Questions
Exam 24: Environmental and Industrial Microbiology88 Questions
Select questions type
The chemical symbol for ________ is ________. (Select all that apply)
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B,C
Two adjacent nitrogen atoms share 3 pairs of electrons resulting in the formation of ________.
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
B
Chemical equilibrium describes ________.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
Isotopes of an element differ from each other by the ________.
(Multiple Choice)
4.9/5  (31)
(31)
Because of its extreme hydrogen bonding, water has a high specific heat so it ________. (Select all that apply)
(Multiple Choice)
4.7/5  (43)
(43)
To minimize the pH change when an acid or base is added to a solution, a ________ is needed.
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following elements is a bacterial micronutrient?
(Multiple Choice)
4.9/5  (44)
(44)
If the pH of a solution is increased from pH 5 to pH 7, it means that the concentration of H+ is ________ than it was at pH 5.
(Multiple Choice)
4.7/5  (38)
(38)
Elements with similar chemical properties are organized in the same ________ of the periodic table.
(Multiple Choice)
4.7/5  (47)
(47)
Weak electrostatic attraction between a hydrogen atom participating in a polar covalent bond and a slightly negative region of a molecule results in the formation of a(n) ________.
(Multiple Choice)
4.8/5  (34)
(34)
Radioisotopes of an element ________. (Select all that apply)
(Multiple Choice)
4.9/5  (32)
(32)
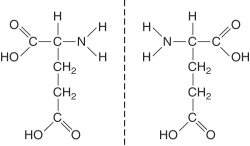
The figure below illustrates ________ isomers. (Select all that apply)
(Multiple Choice)
4.8/5  (28)
(28)
Showing 1 - 20 of 37
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)