Deck 6: Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/31
Play
Full screen (f)
Deck 6: Solids
1
Which of the following statements describe properties of metallic solids? Please select all that apply.
A) good thermal conductivity
B) high density
C) brittle
D) good electrical conductivity
A) good thermal conductivity
B) high density
C) brittle
D) good electrical conductivity
A, B, D
2
Ionic solids are held together with __________ interactions.
electrostatic
3
Match the material with the bonding type.
-platinum (Pt)
A) metallic
B) molecular
C) covalent
D) ionic
-platinum (Pt)
A) metallic
B) molecular
C) covalent
D) ionic
A
4
Match the material with the bonding type.
-N2O
A) metallic
B) molecular
C) covalent
D) ionic
-N2O
A) metallic
B) molecular
C) covalent
D) ionic

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
5
Match the material with the bonding type.
-S
A) metallic
B) molecular
C) covalent
D) ionic
-S
A) metallic
B) molecular
C) covalent
D) ionic

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
6
Match the material with the bonding type.
-SrF2
A) metallic
B) molecular
C) covalent
D) ionic
-SrF2
A) metallic
B) molecular
C) covalent
D) ionic

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
7
A crystalline solid demonstrates long-range order which persists over distances much greater than the bond length between the atoms. It is possible to predict the position of every atom from those given in the unit cell.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
8
The structure of C60 is analogous to the stitching on a European football and is shown in the diagram below. Each carbon lies on the surface of a sphere and the carbon is sp3 hybridized?



Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
9
Silicon dioxide exists as different polymorphs e.g. quartz and cristobalite. When silicon dioxide is melted and quenched (rapidly cooled), it forms an _______ structure known as a glass.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
10
A magnetic levitation train uses the Meissner effect to propel the train along magnetic rails. Which of these facts relating to superconductors are true. Please select all that apply.
A) Superconductors are diamagnetic.
B) No matter the temperature, superconductors exhibit the Meissner effect.
C) Electrons move in pairs through the lattice.
D) The Meissner effect occurs at low temperature when the superconductor is below its critical temperature.
A) Superconductors are diamagnetic.
B) No matter the temperature, superconductors exhibit the Meissner effect.
C) Electrons move in pairs through the lattice.
D) The Meissner effect occurs at low temperature when the superconductor is below its critical temperature.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
11
The structure with repeat sequence ABCCABCCABCCABC is close-packed.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
12
A body centred cubic unit cell is close-packed.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
13
-tungsten crystallises with a body centred cubic structure where the length of the unit cell is 3.150 Å. Use the diagram to calculate the radius of a tungsten atom. (Hint: The radius of the atom is just half the distance between the two closest atoms.)
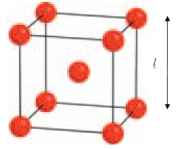
A) 2.692 Å
B) 1.136 Å
C) 1.346 Å
D) 1.575 Å

A) 2.692 Å
B) 1.136 Å
C) 1.346 Å
D) 1.575 Å

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
14
A lattice point on the edge of a cubic unit cell is worth one quarter of a lattice point

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
15
A semiconductor has which of the following properties? Please select all that apply.
A) A filled conduction band.
B) A significant but small band gap.
C) A filled valence band.
D) Conductivity that falls as the temperature falls.
A) A filled conduction band.
B) A significant but small band gap.
C) A filled valence band.
D) Conductivity that falls as the temperature falls.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
16
What is the formula of a compound based on cubic close packing where one quarter of the tetrahedral holes (T) are filled in a lattice made of lattice points of type A. What is the coordination number of the filled holes and the lattice points? Please select all that apply.
A) Formula A2T
B) Coordination number of the lattice point would be 2 and the filled hole would be four.
C) Formula AT2
D) Coordination number of the lattice point would be 4 and the filled hole would be four
A) Formula A2T
B) Coordination number of the lattice point would be 2 and the filled hole would be four.
C) Formula AT2
D) Coordination number of the lattice point would be 4 and the filled hole would be four

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
17
Li2O crystallises with the antifluorite structure where the positions of the cations and anions are in the opposite positions to the structure observed for fluorite. What are the coordination numbers of the lithium and oxygen ions?
A) Lithium is four coordinate and oxygen is six coordinate.
B) Lithium is four coordinate and oxygen is eight coordinate.
C) Lithium is eight coordinate and oxygen is four coordinate.
D) Lithium and oxygen are both six coordinate.
A) Lithium is four coordinate and oxygen is six coordinate.
B) Lithium is four coordinate and oxygen is eight coordinate.
C) Lithium is eight coordinate and oxygen is four coordinate.
D) Lithium and oxygen are both six coordinate.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
18
Lattice point counting for sphalerite shows that the structure contains ___ Zn/S ions.
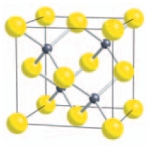


Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
19
The coordination number of iodine in cadmium iodide is six.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
20
There is no structural analogue of CaF2 in hexagonal close packing (fill all tetrahedral holes).

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
21
The structure of the perovskite (calcium titanate) is given below. What is the coordination number of the two cations? Please select all that apply.
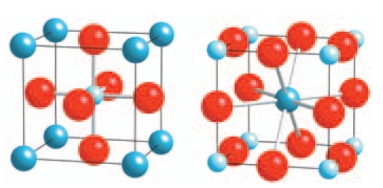
A) Ca is 12 coordinate
B) Ti is 12 coordinate
C) Ti is 6 coordinate
D) Ca is 6 coordinate

A) Ca is 12 coordinate
B) Ti is 12 coordinate
C) Ti is 6 coordinate
D) Ca is 6 coordinate

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
22
The ionic radius of an atom can be measured by X-ray diffraction.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
23
Magnesium oxide crystallizes with the sodium chloride structure. Magnesium oxide obeys the radius ratio rule for this structure.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
24
Use the following data to construct a thermochemical cycle for the formation of ZnS from its elements and use the cycle to calculate the enthalpy of formation of ZnS.
Zn (s) Zn (g) 130 kJ mol-1
Zn (g) Zn+ (g) 906 kJ mol-1
Zn+ (g) Zn2+ (g) 1733 kJ mol-1
ZnS (s) Zn2+ (g) + S2- (g) 3048 kJ mol-1
S (s) S (g) 233 kJ mol-1
S (s) S- (g) -200 kJ mol-1
S- (s) S2- (g) 456 kJ mol-1
A) 200 kJ mol-1
B) -200 kJ mol-1
C) 33 kJ mol-1
D) 2848 kJ mol-1
Zn (s) Zn (g) 130 kJ mol-1
Zn (g) Zn+ (g) 906 kJ mol-1
Zn+ (g) Zn2+ (g) 1733 kJ mol-1
ZnS (s) Zn2+ (g) + S2- (g) 3048 kJ mol-1
S (s) S (g) 233 kJ mol-1
S (s) S- (g) -200 kJ mol-1
S- (s) S2- (g) 456 kJ mol-1
A) 200 kJ mol-1
B) -200 kJ mol-1
C) 33 kJ mol-1
D) 2848 kJ mol-1

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
25
Match the value of Born exponent with the appropriate compound. given that the Born exponent values for the individual ions are Be2+ = 5, F- = 7, Zn2+, S2- and Cl- = 9, Rb+ = 10, Cs+ = 12 respectively.
-ZnS
A) 9.0
B) 10.5
C) 6.33
D) 9.5
-ZnS
A) 9.0
B) 10.5
C) 6.33
D) 9.5

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
26
Match the value of Born exponent with the appropriate compound. given that the Born exponent values for the individual ions are Be2+ = 5, F- = 7, Zn2+, S2- and Cl- = 9, Rb+ = 10, Cs+ = 12 respectively.
-CsCl
A) 9.0
B) 10.5
C) 6.33
D) 9.5
-CsCl
A) 9.0
B) 10.5
C) 6.33
D) 9.5

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
27
Match the value of Born exponent with the appropriate compound. given that the Born exponent values for the individual ions are Be2+ = 5, F- = 7, Zn2+, S2- and Cl- = 9, Rb+ = 10, Cs+ = 12 respectively.
-BeF2
A) 9.0
B) 10.5
C) 6.33
D) 9.5
-BeF2
A) 9.0
B) 10.5
C) 6.33
D) 9.5

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
28
Match the value of Born exponent with the appropriate compound. given that the Born exponent values for the individual ions are Be2+ = 5, F- = 7, Zn2+, S2- and Cl- = 9, Rb+ = 10, Cs+ = 12 respectively.
-RbCl
A) 9.0
B) 10.5
C) 6.33
D) 9.5
-RbCl
A) 9.0
B) 10.5
C) 6.33
D) 9.5

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
29
Use the Born Lande equation to calculate the lattice energy of MgO given that r = 210.5 pm.
A) 7914 kJ mol-1
B) 3957 kJ mol-1
C) 4617 kJ mol-1
D) 989 kJ mol-1
A) 7914 kJ mol-1
B) 3957 kJ mol-1
C) 4617 kJ mol-1
D) 989 kJ mol-1

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
30
Use the Kapustinskii equation to estimate the lattice enthalpy for KBr given that the ionic radii are 138 and 196 pm for K+ and Br- respectively.
A) 646 kJ mol-1
B) 323 kJ mol-1
C) 2584 kJ mol-1
D) 46 kJ mol-1
A) 646 kJ mol-1
B) 323 kJ mol-1
C) 2584 kJ mol-1
D) 46 kJ mol-1

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
31
Iron and cobalt both have similar low electronegativities. The predicted structure based on this information is an alloy.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck


