Exam 6: Solids
Exam 1: Fundamentals57 Questions
Exam 2: The Language of Organic Chemistry25 Questions
Exam 3: Atomic Structure and Properties34 Questions
Exam 4: Diatomic Molecules27 Questions
Exam 5: Polyatomic Molecules30 Questions
Exam 6: Solids31 Questions
Exam 7: Acids and Bases27 Questions
Exam 8: Gases38 Questions
Exam 9: Reaction Kinetics50 Questions
Exam 10: Molecular Spectroscopy25 Questions
Exam 11: Analytical Chemistry24 Questions
Exam 12: Molecular Characterisation30 Questions
Exam 13: Energy and Thermochemistry47 Questions
Exam 14: Entropy and Gibbs Energy40 Questions
Exam 16: Electrochemistry24 Questions
Exam 17: Phase Equilibrium and Solutions25 Questions
Exam 18: Isomerism and Stereochemistry25 Questions
Exam 19: Organic Reaction Mechanisms28 Questions
Exam 20: Halogenoalkanes: Substitution and Elimination Reactions31 Questions
Exam 21: Alkenes and Alkynes: Electrophilic Addition and Pericyclic Reactions27 Questions
Exam 22: Benzene and Other Aromatic Compounds: Electrophilic Substitution Reactions37 Questions
Exam 23: Aldehydes and Ketones: Nucleophilic Addition and Α-Substitution Reactions33 Questions
Exam 24: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution and Α-Substitution Reactions25 Questions
Exam 25: Hydrogen30 Questions
Exam 26: S-Block Chemistry28 Questions
Exam 27: P-Block Chemistry33 Questions
Exam 28: D-Block Chemistry34 Questions
Select questions type
Silicon dioxide exists as different polymorphs e.g. quartz and cristobalite. When silicon dioxide is melted and quenched (rapidly cooled), it forms an _______ structure known as a glass.
Free
(Short Answer)
4.8/5  (40)
(40)
Correct Answer:
amorphous
A semiconductor has which of the following properties? Please select all that apply.
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
B, C, D
Which of the following statements describe properties of metallic solids? Please select all that apply.
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
A, B, D
The structure with repeat sequence ABCCABCCABCCABC is close-packed.
(True/False)
4.7/5  (38)
(38)
The structure of C60 is analogous to the stitching on a European football and is shown in the diagram below. Each carbon lies on the surface of a sphere and the carbon is sp3 hybridized?

(True/False)
4.8/5  (49)
(49)
A magnetic levitation train uses the Meissner effect to propel the train along magnetic rails. Which of these facts relating to superconductors are true. Please select all that apply.
(Multiple Choice)
4.8/5  (36)
(36)
Iron and cobalt both have similar low electronegativities. The predicted structure based on this information is an alloy.
(True/False)
4.8/5  (39)
(39)
What is the formula of a compound based on cubic close packing where one quarter of the tetrahedral holes (T) are filled in a lattice made of lattice points of type A. What is the coordination number of the filled holes and the lattice points? Please select all that apply.
(Multiple Choice)
4.7/5  (39)
(39)
Match the value of Born exponent with the appropriate compound. given that the Born exponent values for the individual ions are Be2+ = 5, F- = 7, Zn2+, S2- and Cl- = 9, Rb+ = 10, Cs+ = 12 respectively.
-RbCl
(Multiple Choice)
4.7/5  (35)
(35)
Lattice point counting for sphalerite shows that the structure contains ___ Zn/S ions.
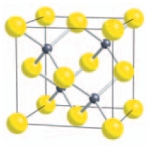
(Short Answer)
4.8/5  (34)
(34)
The ionic radius of an atom can be measured by X-ray diffraction.
(True/False)
4.8/5  (42)
(42)
Li2O crystallises with the antifluorite structure where the positions of the cations and anions are in the opposite positions to the structure observed for fluorite. What are the coordination numbers of the lithium and oxygen ions?
(Multiple Choice)
4.8/5  (40)
(40)
A lattice point on the edge of a cubic unit cell is worth one quarter of a lattice point
(True/False)
4.9/5  (29)
(29)
-tungsten crystallises with a body centred cubic structure where the length of the unit cell is 3.150 Å. Use the diagram to calculate the radius of a tungsten atom. (Hint: The radius of the atom is just half the distance between the two closest atoms.)
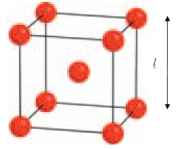
(Multiple Choice)
4.8/5  (44)
(44)
Match the value of Born exponent with the appropriate compound. given that the Born exponent values for the individual ions are Be2+ = 5, F- = 7, Zn2+, S2- and Cl- = 9, Rb+ = 10, Cs+ = 12 respectively.
-ZnS
(Multiple Choice)
4.8/5  (26)
(26)
A crystalline solid demonstrates long-range order which persists over distances much greater than the bond length between the atoms. It is possible to predict the position of every atom from those given in the unit cell.
(True/False)
4.9/5  (28)
(28)
Showing 1 - 20 of 31
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)