Deck 5: Polyatomic Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 5: Polyatomic Molecules
1
The 'formal charge' is defined as?
A) The number of valence electrons for an atom.
B) The difference between valence electrons in the free atom and the number of atoms assigned to that atom in its Lewis structure.
C) The highest oxidation state expected in a particular group.
D) The most common oxidation state of a particular atom.
A) The number of valence electrons for an atom.
B) The difference between valence electrons in the free atom and the number of atoms assigned to that atom in its Lewis structure.
C) The highest oxidation state expected in a particular group.
D) The most common oxidation state of a particular atom.
B
2
In the second period from Li to Ne, while no molecules exist which have more than eight electrons, there are some which exist with less. These molecules are described as electron ____
poor
3
COS adopts a structure with a central carbon atom.
True
4
When negative charges are present in a structure where formal charges can be assigned, the lowest energy structure is the one with the negative charge on the most ____________ atom.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following factors affect the shape at the central atom in VSEPR theory? Please select all that apply.
A) The charge.
B) The bonded electron pairs.
C) The bonded electron pairs.
D) The electronic contribution of the ligands.
A) The charge.
B) The bonded electron pairs.
C) The bonded electron pairs.
D) The electronic contribution of the ligands.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
When predicting the shape of a molecule containing lone pairs, which of the following should be arranged first?
A) lone pairs
B) double bonds
C) single bonds
D) triple bonds
A) lone pairs
B) double bonds
C) single bonds
D) triple bonds

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following geometries are predicted for molecules in which all the electron pairs are bonding in VSEPR theory? Please select all that apply.
A) linear
B) tetrahedral
C) square pyramidal
D) pentagonal bipyramidal
A) linear
B) tetrahedral
C) square pyramidal
D) pentagonal bipyramidal

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
In VSEPR theory, the most important interactions that should be considered when placing bonding and lone pairs of electrons in a trigonal bipyramid occur at 90°.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
Use VSEPR theory to match the molecule with its shape.
-[ClF4]-
A) square planar
B) angular
C) pyramidal
D) tetrahedral
-[ClF4]-
A) square planar
B) angular
C) pyramidal
D) tetrahedral

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
Use VSEPR theory to match the molecule with its shape.
-H2S
A) square planar
B) angular
C) pyramidal
D) tetrahedral
-H2S
A) square planar
B) angular
C) pyramidal
D) tetrahedral

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
Use VSEPR theory to match the molecule with its shape.
-PH3
A) square planar
B) angular
C) pyramidal
D) tetrahedral
-PH3
A) square planar
B) angular
C) pyramidal
D) tetrahedral

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
Use VSEPR theory to match the molecule with its shape.
-CCl2(CH3)2
A) square planar
B) angular
C) pyramidal
D) tetrahedral
-CCl2(CH3)2
A) square planar
B) angular
C) pyramidal
D) tetrahedral

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
The shape predicted by VSEPR theory for [BrF4]+ is trigonal pyramidal.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
Using VSEPR theory, a molecule having 5 electron pairs divided between 3 bonding pairs and 2 lone pairs will be trigonal planar true-false

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
A double bond takes up less room than a single bond. true-false

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
Use VSEPR theory to determine the structure of the borate anion, [BO4]5-.
A) Trigonal bipyramidal with one lone pair and four bond pairs.
B) Trigonal bipyramidal with two lone pairs and three bond pairs.
C) Tetrahedral
D) Square planar
A) Trigonal bipyramidal with one lone pair and four bond pairs.
B) Trigonal bipyramidal with two lone pairs and three bond pairs.
C) Tetrahedral
D) Square planar

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
VSEPR predicts that shape of the ion TeBr62- is octahedral.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
The linear molecule COS is non polar.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
A stream of trichloromethane (chloroform) will be deflected by a positive charge because the more electronegative ____ atoms generate dipoles which do not cancel each other out.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
The process of an atom changing the energy and shape of its atomic orbitals to increase the opportunities for bonding with other atoms is called __________

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
BeMe2 rapidly polymerizes to form a linear chain of tetrahedral Be atoms. The same process does not occur for BeBut2 due to steric hindrance and the shape at Be is linear. What sort of hybridization is present in each case?
A) BeMe2 is sp2 hybridized and BeBut2 is sp hybridized.
B) BeMe2is sp3 hybridized and BeBut2 is sp hybridized.
C) BeMe2is sp3 hybridized and BeBut2 is sp2 hybridized.
D) BeMe2is sp hybridized and BeBut2 is sp3 hybridized.
A) BeMe2 is sp2 hybridized and BeBut2 is sp hybridized.
B) BeMe2is sp3 hybridized and BeBut2 is sp hybridized.
C) BeMe2is sp3 hybridized and BeBut2 is sp2 hybridized.
D) BeMe2is sp hybridized and BeBut2 is sp3 hybridized.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
For each of the atoms highlighted in bold, determine the type of hybridization present.
-CH3CH2CCH
A) sp
B) sp2
C) sp3
-CH3CH2CCH
A) sp
B) sp2
C) sp3

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
For each of the atoms highlighted in bold, determine the type of hybridization present.
-C6H5CO2H (benzoic acid)
A) sp
B) sp2
C) sp3
-C6H5CO2H (benzoic acid)
A) sp
B) sp2
C) sp3

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
For each of the atoms highlighted in bold, determine the type of hybridization present.
-CH3CH2OH
A) sp
B) sp2
C) sp3
-CH3CH2OH
A) sp
B) sp2
C) sp3

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
SF6 is a hypervalent compound of sulfur which lies in the third period of the Periodic Table. Charge separation can be use to account for the exceptional valence of SF6.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
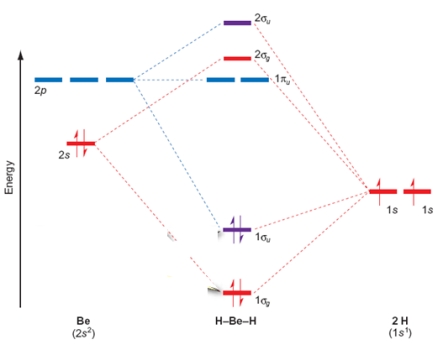
In the BeH2 molecular orbital diagram shown below, the Be is double bonded to the H atoms.


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
In BeH2, the 2g orbital is closer in energy and properties to the atomic orbitals of _______

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
The total number of electrons surrounding Xe in XeF4 as predicted by VSEPR theory is 12. Valence bond theory would suggest that this arrangement would require d2sp3 hybridization.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
One way of describing the bonding in XeF2 which avoids hypervalency for xenon is using the 3 centre 4 electron bond. Which combination of orbitals generate a non-bonding combination for the XeF2 molecule?
A) In phase combination of the two F pz orbitals and the pz orbital on the Xe.
B) Out of phase combination of the two F pz orbitals and the pz orbital on the Xe.
C) In phase combination of the two F pz orbitals and the px orbital on the Xe.
D) In phase combination of the two F px orbitals and the pz orbital on the Xe.
A) In phase combination of the two F pz orbitals and the pz orbital on the Xe.
B) Out of phase combination of the two F pz orbitals and the pz orbital on the Xe.
C) In phase combination of the two F pz orbitals and the px orbital on the Xe.
D) In phase combination of the two F px orbitals and the pz orbital on the Xe.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
The bonding in B2H6 is best described by which of the following statements?
A) 2-centre 2-electron bonds, 3-centre 2-electron bonding and 2-centre 2-electron bonding
B) 3-centre 2-electron bonding and 2-centre 2-electron bonding
C) 3-centre 4-electron bonds
D) 3-centre 2-electron bonding
A) 2-centre 2-electron bonds, 3-centre 2-electron bonding and 2-centre 2-electron bonding
B) 3-centre 2-electron bonding and 2-centre 2-electron bonding
C) 3-centre 4-electron bonds
D) 3-centre 2-electron bonding

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


