Deck 9: The Chemistry of Alkyl Halides
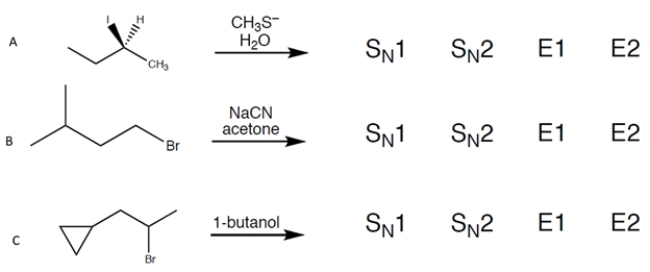
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 9: The Chemistry of Alkyl Halides
1
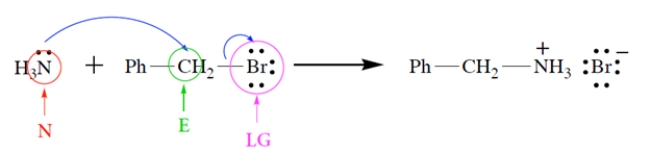
Consider the reaction:
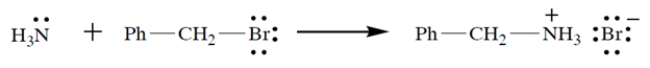 a. On the left side of the equation, draw the curved-arrow notation for the reaction in the left-to-right direction.
a. On the left side of the equation, draw the curved-arrow notation for the reaction in the left-to-right direction.
b. On the left side of the equation, circle the nucleophile and label it "N."
c. On the left side of the equation, circle the electrophilic atom and label it "E."
d. On the left side of the equation, circle the leaving group and label it "LG."
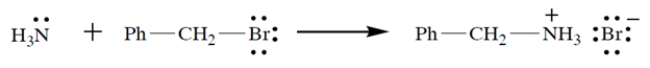 a. On the left side of the equation, draw the curved-arrow notation for the reaction in the left-to-right direction.
a. On the left side of the equation, draw the curved-arrow notation for the reaction in the left-to-right direction.b. On the left side of the equation, circle the nucleophile and label it "N."
c. On the left side of the equation, circle the electrophilic atom and label it "E."
d. On the left side of the equation, circle the leaving group and label it "LG."
2
(a) Provide the structure of the missing starting material. (b) Then, assuming the reaction occurs via an SN2 mechanism, provide the rate law using the appropriate concentration terms.
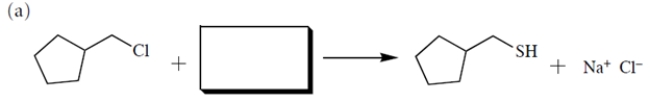

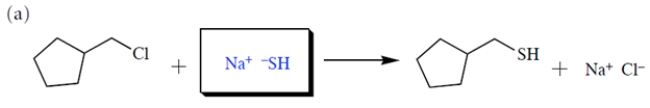 (b) By definition, the SN2 reaction is a bimolecular process, and the rate law contains concentration terms for both the alkyl halide and the nucleophile.
(b) By definition, the SN2 reaction is a bimolecular process, and the rate law contains concentration terms for both the alkyl halide and the nucleophile.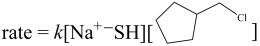
3
The rate law for this reaction is shown. Draw a circle around the alkyl halide that would have the highest value for the rate constant, k. underline the alkyl halide with the lowest value for k.
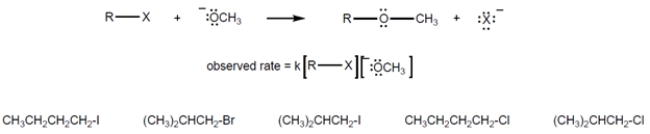

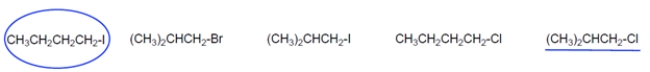
4
Circle all possible products for the reaction:
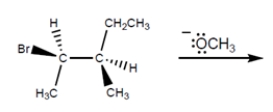



Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
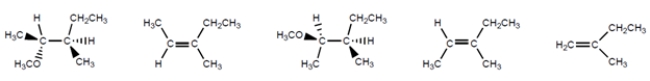
Circle the reasonable mechanisms that these reactions will follow. (There may be more than one.)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
Complete the SN2 reaction on (R)-2-iodobutane and clearly depict any stereochemistry.
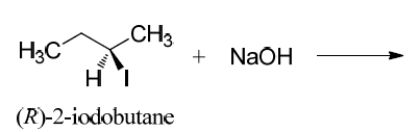


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
Rank the compounds in order of greatest reactivity to the SN2 reaction, where 1 is the most reactive and 4 is the least reactive.
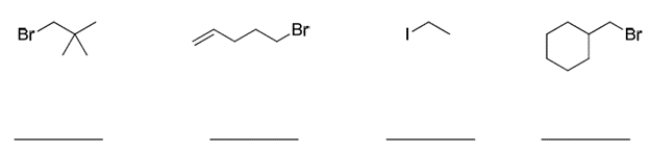


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
What are the two products that form during an E2 reaction on (1-bromoethyl)-cyclohexane?
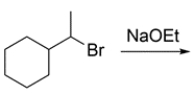
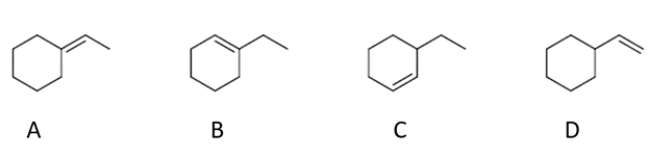
A) compound A
B) compound B
C) compound C
D) compound D


A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Write the mechanism of the reaction (that is, SN2, SN1, E2, E1) that is required to form each product in the reaction:
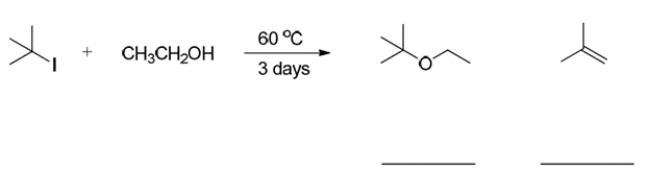


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Give the structure of the nucleophile that would give the product shown in the SN2 reaction. Show any charges or unshared valence electrons. Then give the curved-arrow notation for the reaction. (Show it on the diagram.)
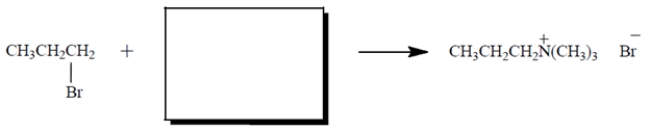


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Under what conditions would the reaction be most likely to proceed to the product shown at a reasonable rate? (Select one.)
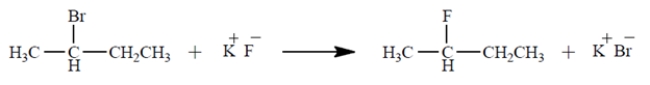
A) in a hydrocarbon solvent such as hexane
B) in ethanol solvent
C) in an ethanol solvent containing some water
D) in very dry dimethyl sulfoxide (DMSO, a polar aprotic solvent) containing a K+-bonding crown ether
E) in dimethyl sulfoxide containing a trace of water and a K+-bonding crown etherf.
in water containing enough acetone to dissolve the alkyl halide, plus a K+-bonding crown ether

A) in a hydrocarbon solvent such as hexane
B) in ethanol solvent
C) in an ethanol solvent containing some water
D) in very dry dimethyl sulfoxide (DMSO, a polar aprotic solvent) containing a K+-bonding crown ether
E) in dimethyl sulfoxide containing a trace of water and a K+-bonding crown etherf.
in water containing enough acetone to dissolve the alkyl halide, plus a K+-bonding crown ether

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Which compound will undergo the fastest SN1/E1 reaction in methanol, a protic solvent?
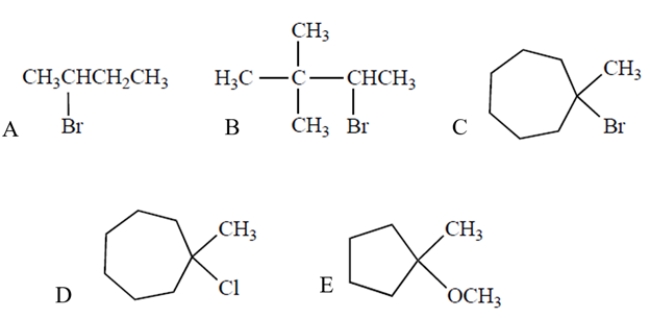
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Write the two possible -elimination products formed in the reaction:
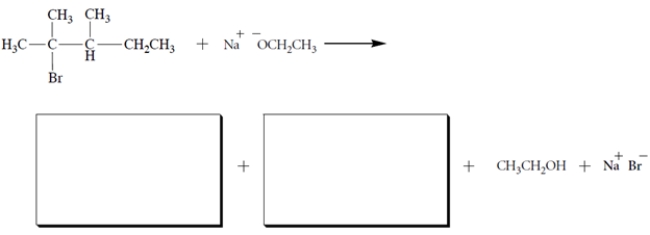


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Predict the organic product(s) of the reaction and give the shorthand for mechanism (that is, E1, E2, SN1, SN2) by which each is formed.
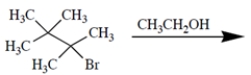
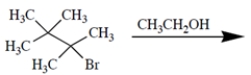

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
According to the rate law for the SN2 reaction, doubling the nucleophile concentration (without changing the concentration of the electrophilic compound) should (select one)
A) decrease the reaction rate.
B) increase the reaction rate by a factor of √2.
C) increase the reaction rate by a factor of 2.
D) increase the reaction rate by a factor of 22, or 4.
E) increase the reaction rate by some other factor.
A) decrease the reaction rate.
B) increase the reaction rate by a factor of √2.
C) increase the reaction rate by a factor of 2.
D) increase the reaction rate by a factor of 22, or 4.
E) increase the reaction rate by some other factor.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
When we say, "sodium ethoxide (Na+ EtO−) acts as a nucleophile," we always mean
A) that the sodium ion accepts a pair of electrons from the electrophile.
B) that the oxygen donates a pair of electrons to a carbon atom.
C) that the oxygen donates a pair of electrons to a leaving group.
D) that the oxygen donates a pair of electrons to an electrophile other than hydrogen.
E) that the leaving group donates a pair of electrons to the electrophile.
A) that the sodium ion accepts a pair of electrons from the electrophile.
B) that the oxygen donates a pair of electrons to a carbon atom.
C) that the oxygen donates a pair of electrons to a leaving group.
D) that the oxygen donates a pair of electrons to an electrophile other than hydrogen.
E) that the leaving group donates a pair of electrons to the electrophile.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Which compound will be most reactive in the SN2 reaction with EtO− Na+ in EtOH?
A) CH3CH2Br
B) (CH3)2CHBr
C) (CH3)3CCH2Br
D) (CH3)3CBr
E) CH3CH2I
A) CH3CH2Br
B) (CH3)2CHBr
C) (CH3)3CCH2Br
D) (CH3)3CBr
E) CH3CH2I

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Consider this reaction:
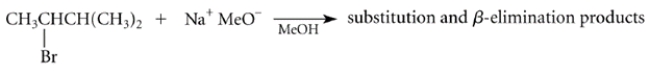 Draw the structures of the two -elimination products that can be formed in this reaction.
Draw the structures of the two -elimination products that can be formed in this reaction.
 Draw the structures of the two -elimination products that can be formed in this reaction.
Draw the structures of the two -elimination products that can be formed in this reaction.
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Which species would act as the best (most reactive) nucleophile toward methyl iodide in a polar aprotic solvent?
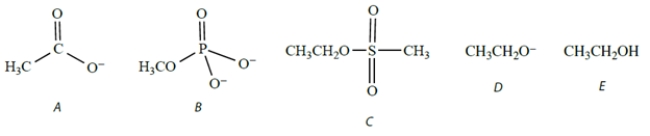
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Give the two organic products of the reaction. (Ignore stereochemistry.)
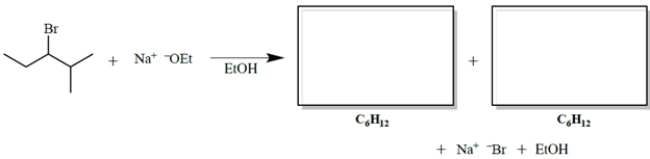


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Which compound undergoes the fastest SN2 reaction with Na+ CH3O−?
A) CH3CH2Cl
B) (CH3)2CHCH2Br
C) (CH3)3CBr
D) CH3CH2Br
E) CH3CH2F
A) CH3CH2Cl
B) (CH3)2CHCH2Br
C) (CH3)3CBr
D) CH3CH2Br
E) CH3CH2F

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Which compound undergoes the fastest SN1 solvolysis reaction with water in acetone?
A) CH3CH2Cl
B) (CH3)2CHCH2Br
C) (CH3)3CBr
D) CH3CH2Br
E) CH3CH2F
A) CH3CH2Cl
B) (CH3)2CHCH2Br
C) (CH3)3CBr
D) CH3CH2Br
E) CH3CH2F

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Which of these compounds will undergo the most rapid solvolysis reaction in methanol? (No base is added.)
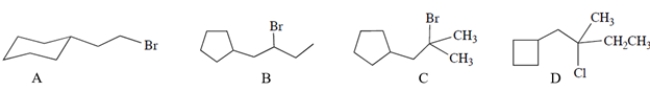
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Rank the starting materials in terms of their rate of product formation in a reaction with sodium fluoride in dimethylformamide (1 being the fastest and 5 being the slowest). (Dimethylformamide (DMF) is a polar aprotic solvent.)
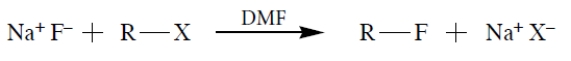
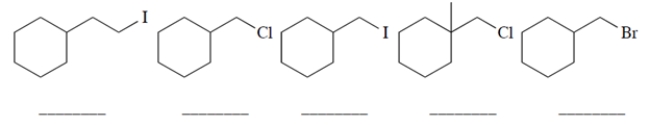



Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
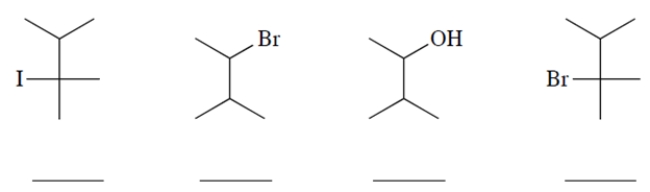
Rank these starting materials on their rate of reaction in hot ethanol (1 being the fastest and 4 being the slowest).


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


