Exam 9: The Chemistry of Alkyl Halides
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Consider the reaction:
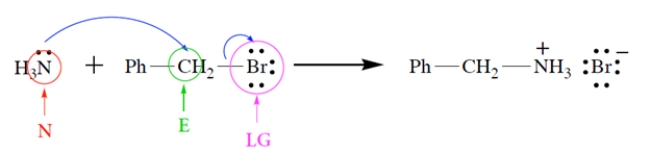
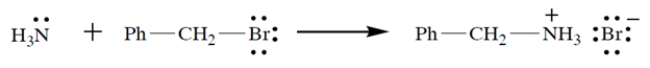 a. On the left side of the equation, draw the curved-arrow notation for the reaction in the left-to-right direction.
b. On the left side of the equation, circle the nucleophile and label it "N."
c. On the left side of the equation, circle the electrophilic atom and label it "E."
d. On the left side of the equation, circle the leaving group and label it "LG."
a. On the left side of the equation, draw the curved-arrow notation for the reaction in the left-to-right direction.
b. On the left side of the equation, circle the nucleophile and label it "N."
c. On the left side of the equation, circle the electrophilic atom and label it "E."
d. On the left side of the equation, circle the leaving group and label it "LG."
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
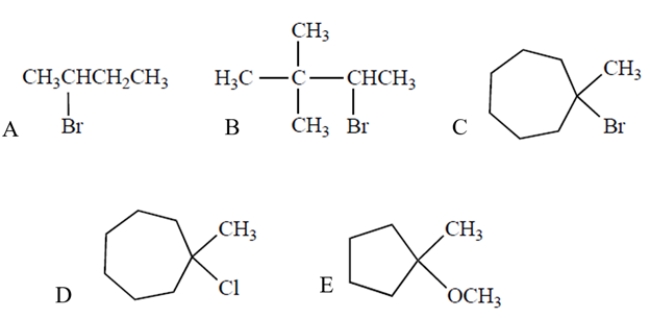
Which compound will undergo the fastest SN1/E1 reaction in methanol, a protic solvent?
Free
(Multiple Choice)
4.8/5  (23)
(23)
Correct Answer:
C
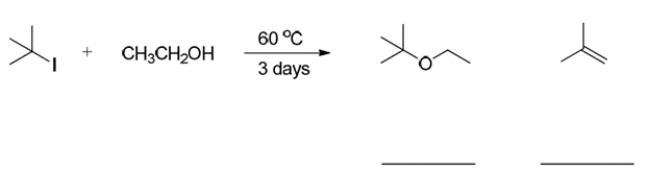
Write the mechanism of the reaction (that is, SN2, SN1, E2, E1) that is required to form each product in the reaction:
Free
(Essay)
4.8/5  (25)
(25)
Correct Answer:
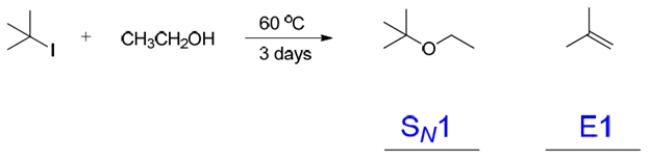
Predict the organic product(s) of the reaction and give the shorthand for mechanism (that is, E1, E2, SN1, SN2) by which each is formed.
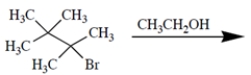
(Essay)
4.8/5  (32)
(32)
Which compound undergoes the fastest SN1 solvolysis reaction with water in acetone?
(Multiple Choice)
4.9/5  (33)
(33)
Which compound undergoes the fastest SN2 reaction with Na+ CH3O−?
(Multiple Choice)
4.8/5  (35)
(35)
Which compound will be most reactive in the SN2 reaction with EtO− Na+ in EtOH?
(Multiple Choice)
4.9/5  (31)
(31)
Give the structure of the nucleophile that would give the product shown in the SN2 reaction. Show any charges or unshared valence electrons. Then give the curved-arrow notation for the reaction. (Show it on the diagram.)
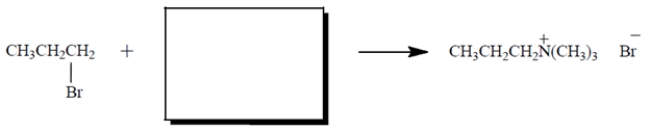
(Essay)
4.9/5  (41)
(41)
Which species would act as the best (most reactive) nucleophile toward methyl iodide in a polar aprotic solvent?
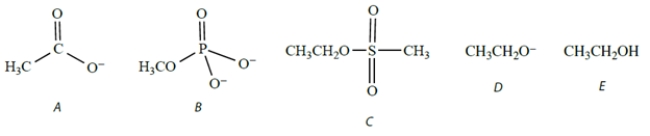
(Multiple Choice)
4.9/5  (45)
(45)
Which of these compounds will undergo the most rapid solvolysis reaction in methanol? (No base is added.)
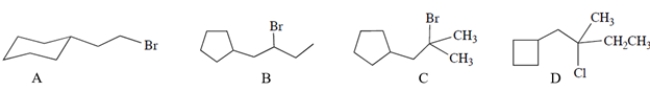
(Multiple Choice)
4.9/5  (40)
(40)
When we say, "sodium ethoxide (Na+ EtO−) acts as a nucleophile," we always mean
(Multiple Choice)
4.9/5  (28)
(28)
Give the two organic products of the reaction. (Ignore stereochemistry.)
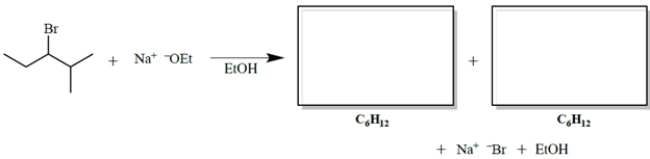
(Essay)
4.7/5  (33)
(33)
Consider this reaction:
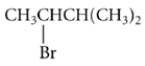 +
Draw the structures of the two -elimination products that can be formed in this reaction.
+
Draw the structures of the two -elimination products that can be formed in this reaction.
(Essay)
4.9/5  (43)
(43)
Rank the starting materials in terms of their rate of product formation in a reaction with sodium fluoride in dimethylformamide (1 being the fastest and 5 being the slowest). (Dimethylformamide (DMF) is a polar aprotic solvent.)
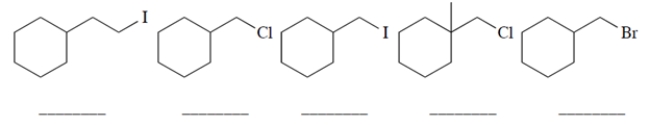
(Essay)
4.9/5  (31)
(31)
Under what conditions would the reaction be most likely to proceed to the product shown at a reasonable rate? (Select one.)
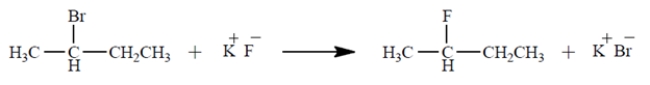
(Multiple Choice)
4.8/5  (39)
(39)
What are the two products that form during an E2 reaction on (1-bromoethyl)-cyclohexane?
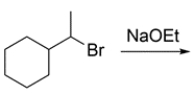
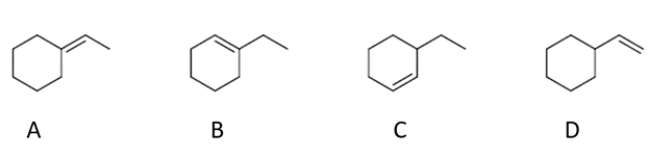
(Multiple Choice)
4.9/5  (30)
(30)
(a) Provide the structure of the missing starting material. (b) Then, assuming the reaction occurs via an SN2 mechanism, provide the rate law using the appropriate concentration terms.
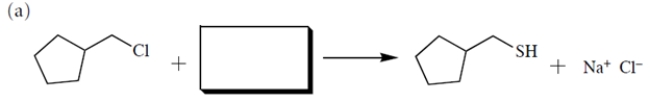
(Essay)
4.8/5  (34)
(34)
According to the rate law for the SN2 reaction, doubling the nucleophile concentration (without changing the concentration of the electrophilic compound) should (select one)
(Multiple Choice)
4.8/5  (28)
(28)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)