Deck 8: Nomenclature and Noncovalent Intermolecular Interactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 8: Nomenclature and Noncovalent Intermolecular Interactions
1
Provide a systematic name for the compound, including any stereochemical designations.
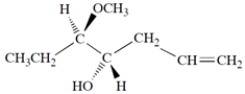

(4S,5R)-5-methoxy-1-hepten-4-ol
2
Name the compound with an IUPAC systematic name.
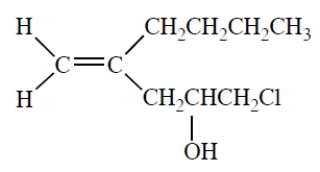

4-butyl-1-chloro-4-penten-2-ol (or 4-butyl-1-chloropent-4-en-2-ol)
3
Select the structure of the compound with the highest boiling point.
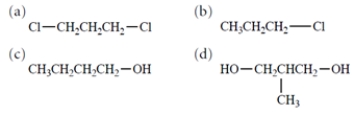
A) compound a
B) compound b
C) compound c
D) compound d
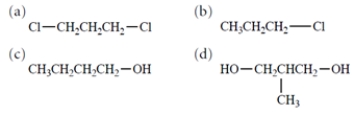
A) compound a
B) compound b
C) compound c
D) compound d
D
4
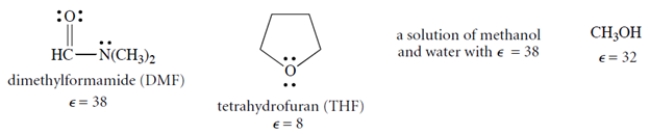
When DMSO dissolves potassium chloride (K+ Cl−), what is the solvation of the salt due to?
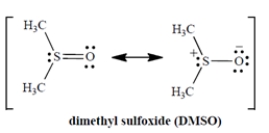
A) The charge-dipole interaction of the sulfur with the K+.
B) The charge-dipole interaction of the oxygen with the K+.
C) The charge-dipole interaction of the oxygen with the Cl−.
D) The hydrogen-bonding interaction of the chloride ion with the C-H bonds.

A) The charge-dipole interaction of the sulfur with the K+.
B) The charge-dipole interaction of the oxygen with the K+.
C) The charge-dipole interaction of the oxygen with the Cl−.
D) The hydrogen-bonding interaction of the chloride ion with the C-H bonds.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
When two hydrocarbon molecules (such as hexane), initially dissolved in water, come together to form an aggregate in water, two of the following are true.
A) The process has ΔG° > 0.
B) The process has ΔH° << 0.
C) The free energy of the process is dominated by the negative entropy of mixing.
D) The process has TΔS° > 0.
E) The process has ΔG° < 0.
A) The process has ΔG° > 0.
B) The process has ΔH° << 0.
C) The free energy of the process is dominated by the negative entropy of mixing.
D) The process has TΔS° > 0.
E) The process has ΔG° < 0.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
Dissolving hexane in water has
A) a large negative ∆S°.
B) a large positive ∆S°.
C) a large negative ∆G°.
D) a large negative ∆H°.
E) a large positive ∆H°.
A) a large negative ∆S°.
B) a large positive ∆S°.
C) a large negative ∆G°.
D) a large negative ∆H°.
E) a large positive ∆H°.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
The boiling point of pentane is 36 °C. The compound with the highest boiling point is
A) water (bp = 100 °C).
B) octane.
C) nonane.
D) 2,2,4,4-tetramethylpentane.
E) methanol.
A) water (bp = 100 °C).
B) octane.
C) nonane.
D) 2,2,4,4-tetramethylpentane.
E) methanol.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Sodium chloride, an ionic compound, should be most soluble in which solvent? ( = dielectric constant)
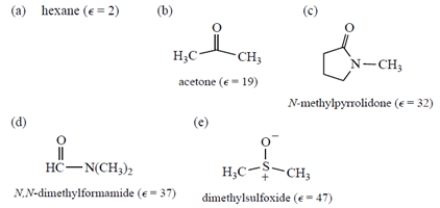
A) compound a
B) compound b
C) compound c
D) compound d
E) compound e

A) compound a
B) compound b
C) compound c
D) compound d
E) compound e

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Arrange these compounds in order of increasing boiling point (lowest boiling point first).
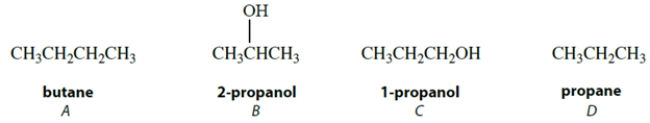
A) A < B < C < D
B) B < C < D < A
C) D < A < C < B
D) D < A < B < C

A) A < B < C < D
B) B < C < D < A
C) D < A < C < B
D) D < A < B < C

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
In each case, identify the better solvent for dissolving the compounds. Choose between H2O (ε = 78) or benzene (ε = 2.3). (Benzene is a six-carbon cyclic hydrocarbon.)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
By circling three items below each structure, classify each of the following solvents as polar or apolar, donor or nondonor, protic or aprotic.
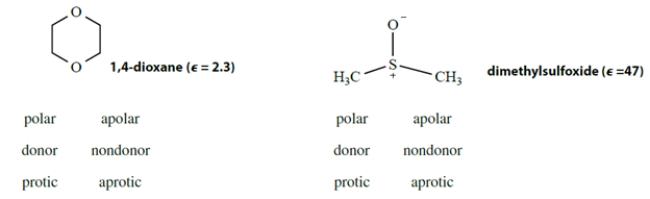


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Which compound would form the strongest complex with potassium ion?
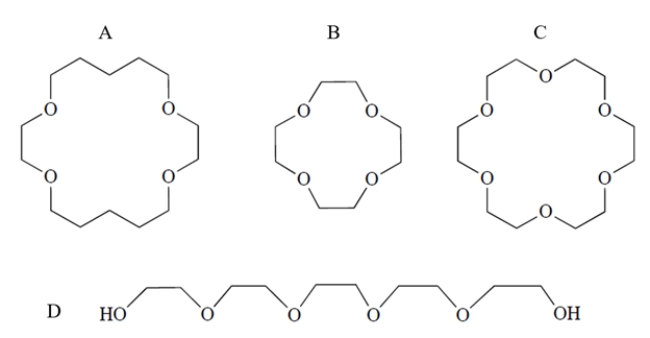
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
In which solvent should NaCl (an ionic compound) have the greatest solubility? (ε = dielectric constant.) Explain your choice briefly.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Until the FDA rescinded the permit for its use in 2002, perflubron, a mixture of 1-bromoperfluorooctyl bromide [CF3(CF2)6CF2Br] and 1-bromoperfluoroodecyl bromide [CF3(CF2)8CF2Br], was being investigated for possible use as artificial blood during surgery. Perflubron dissolves substantial amounts of oxygen; a mouse can breathe when submerged in oxygenated perflubron. Perflubron is completely insoluble in water. To make it compatible with the aqueous biological environment, it had to be suspended in water in such a way that it would form an emulsion and not separate into layers. (In an emulsion, water-insoluble substances are suspended as small particles that do not form a separate layer.) Addition of lecithin, a phospholipid from egg yolks, met this objective.
![Until the FDA rescinded the permit for its use in 2002, perflubron, a mixture of 1-bromoperfluorooctyl bromide [CF<sub>3</sub>(CF<sub>2</sub>)<sub>6</sub>CF<sub>2</sub>Br] and 1-bromoperfluoroodecyl bromide [CF<sub>3</sub>(CF<sub>2</sub>)<sub>8</sub>CF<sub>2</sub>Br], was being investigated for possible use as artificial blood during surgery. Perflubron dissolves substantial amounts of oxygen; a mouse can breathe when submerged in oxygenated perflubron. Perflubron is completely insoluble in water. To make it compatible with the aqueous biological environment, it had to be suspended in water in such a way that it would form an emulsion and not separate into layers. (In an emulsion, water-insoluble substances are suspended as small particles that do not form a separate layer.) Addition of lecithin, a phospholipid from egg yolks, met this objective. Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.](https://storage.examlex.com/TBMC1048/11edaded_7d16_96fe_a31a_03338cb64c69_TBMC1048_00.jpg) Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.
Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.
![Until the FDA rescinded the permit for its use in 2002, perflubron, a mixture of 1-bromoperfluorooctyl bromide [CF<sub>3</sub>(CF<sub>2</sub>)<sub>6</sub>CF<sub>2</sub>Br] and 1-bromoperfluoroodecyl bromide [CF<sub>3</sub>(CF<sub>2</sub>)<sub>8</sub>CF<sub>2</sub>Br], was being investigated for possible use as artificial blood during surgery. Perflubron dissolves substantial amounts of oxygen; a mouse can breathe when submerged in oxygenated perflubron. Perflubron is completely insoluble in water. To make it compatible with the aqueous biological environment, it had to be suspended in water in such a way that it would form an emulsion and not separate into layers. (In an emulsion, water-insoluble substances are suspended as small particles that do not form a separate layer.) Addition of lecithin, a phospholipid from egg yolks, met this objective. Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.](https://storage.examlex.com/TBMC1048/11edaded_7d16_96fe_a31a_03338cb64c69_TBMC1048_00.jpg) Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.
Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Draw the structure of 3-hexyn-2-ol.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Name the compound. Include the appropriate stereochemical designation in the name.
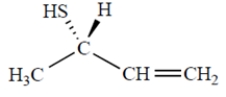


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Name the compound. Include the appropriate stereochemical designation in the name.
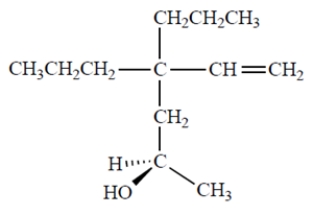


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Draw the structure of 3-ethoxy-2-butanethiol (alternate name 3-ethoxybutane-2-thiol).

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Given four compounds with the same branching pattern and similar molecular masses, which compound has the highest boiling point?
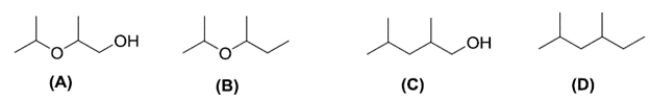
A) compound A
B) compound B
C) compound C
D) compound D
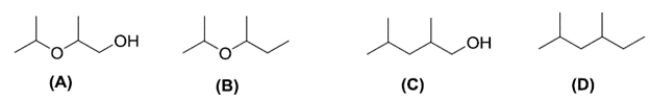
A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Which structure has the lowest melting point of the various 18-carbon fatty acids given?
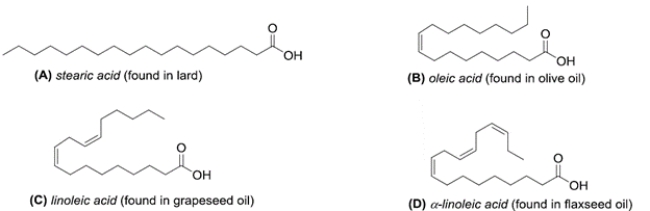
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the equilibria in CCl4, an apolar, aprotic, nondonor solvent.
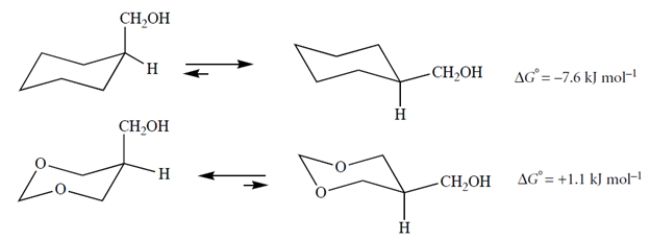 Explain why replacing the two carbons of the ring shown above with oxygens makes the axial conformation more stable.
Explain why replacing the two carbons of the ring shown above with oxygens makes the axial conformation more stable.
 Explain why replacing the two carbons of the ring shown above with oxygens makes the axial conformation more stable.
Explain why replacing the two carbons of the ring shown above with oxygens makes the axial conformation more stable.
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Compounds consisting of molecules that can both donate and accept hydrogen bonds have higher boiling points than isomeric compounds that cannot donate hydrogen bonds because
A) hydrogen bonding increases molecular stability.
B) hydrogen bonding decreases molecular stability, and less-stable molecules have a greater tendency to escape from solution.
C) hydrogen bonding between molecules lowers the vapor pressure, so a higher temperature is required to increase the vapor pressure to atmospheric pressure.
D) hydrogen bonding increases water solubility.
A) hydrogen bonding increases molecular stability.
B) hydrogen bonding decreases molecular stability, and less-stable molecules have a greater tendency to escape from solution.
C) hydrogen bonding between molecules lowers the vapor pressure, so a higher temperature is required to increase the vapor pressure to atmospheric pressure.
D) hydrogen bonding increases water solubility.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Drugs are often conjugated to glucuronic acid groups in biological systems. Explain why you would need to conjugate a drug to glucuronic acid and how glucuronides solve this problem.
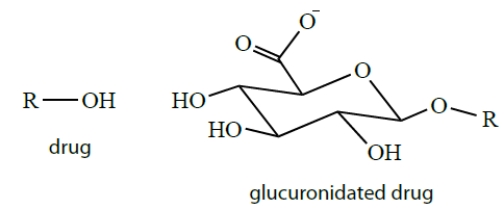


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
This is the predominant form of the sugar D-glucose.
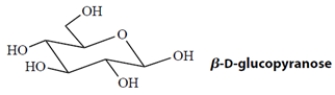 Glucose does not passively cross cell membranes, even though it is a neutral molecule. Explain why.
Glucose does not passively cross cell membranes, even though it is a neutral molecule. Explain why.
 Glucose does not passively cross cell membranes, even though it is a neutral molecule. Explain why.
Glucose does not passively cross cell membranes, even though it is a neutral molecule. Explain why.
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Draw all the alcohols having a molecular formula of C4H10O.
a. Indicate if the alcohol is chiral.
b. Name each alcohol using IUPAC nomenclature.
c. Classify each alcohol as primary, secondary or tertiary.
a. Indicate if the alcohol is chiral.
b. Name each alcohol using IUPAC nomenclature.
c. Classify each alcohol as primary, secondary or tertiary.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


