Exam 8: Nomenclature and Noncovalent Intermolecular Interactions
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
When two hydrocarbon molecules (such as hexane), initially dissolved in water, come together to form an aggregate in water, two of the following are true.
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
D, E
This is the predominant form of the sugar D-glucose.
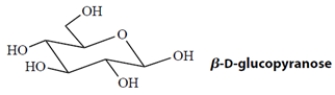 Glucose does not passively cross cell membranes, even though it is a neutral molecule. Explain why.
Glucose does not passively cross cell membranes, even though it is a neutral molecule. Explain why.
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
The cell membrane consists of a lipid bilayer, where the outside of the layer contains the hydrophilic head groups and the inner portion contains the hydrophobic tails. In order to pass through the cell membrane through passive diffusion, the molecule must not be insoluble in hydrocarbons, nor highly soluble in hydrocarbons. Small neutral molecules like molecular oxygen can pass through, but glucose is a much larger molecule. While it contains six carbon atoms, it also contains five hydrophilic hydroxy groups that will not be able to pass through the hydrocarbon tails. In the body, glucose requires carrier proteins that help it to cross cell membranes.
The boiling point of pentane is 36 °C. The compound with the highest boiling point is
Free
(Multiple Choice)
4.7/5  (42)
(42)
Correct Answer:
C
In each case, identify the better solvent for dissolving the compounds. Choose between H2O (ε = 78) or benzene (ε = 2.3). (Benzene is a six-carbon cyclic hydrocarbon.)
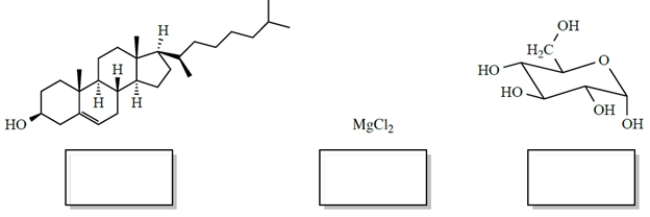
(Short Answer)
4.9/5  (39)
(39)
In which solvent should NaCl (an ionic compound) have the greatest solubility? (ε = dielectric constant.) Explain your choice briefly.
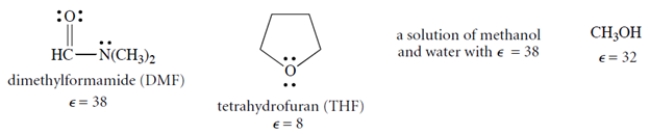
(Essay)
4.8/5  (42)
(42)
Sodium chloride, an ionic compound, should be most soluble in which solvent? ( = dielectric constant)
(a) hexane
(b)
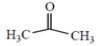 acetone
(c)
acetone
(c)
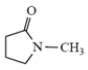 -methylpyrrolidone
(d)
-methylpyrrolidone
(d)
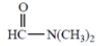 (e)
(e)
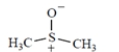 dimethylsulfoxide
dimethylsulfoxide
(Multiple Choice)
4.8/5  (26)
(26)
Name the compound. Include the appropriate stereochemical designation in the name.
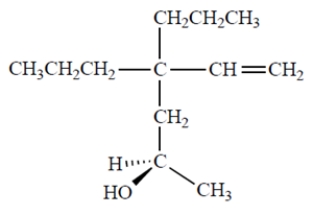
(Short Answer)
4.8/5  (36)
(36)
Consider the equilibria in CCl4, an apolar, aprotic, nondonor solvent.
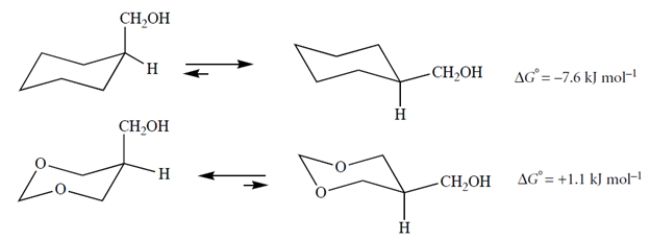 Explain why replacing the two carbons of the ring shown above with oxygens makes the axial conformation more stable.
Explain why replacing the two carbons of the ring shown above with oxygens makes the axial conformation more stable.
(Essay)
4.7/5  (34)
(34)
Draw all the alcohols having a molecular formula of C4H10O.
a. Indicate if the alcohol is chiral.
b. Name each alcohol using IUPAC nomenclature.
c. Classify each alcohol as primary, secondary or tertiary.
(Essay)
4.8/5  (31)
(31)
When DMSO dissolves potassium chloride (K+ Cl−), what is the solvation of the salt due to?
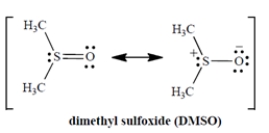
(Multiple Choice)
4.8/5  (28)
(28)
Drugs are often conjugated to glucuronic acid groups in biological systems. Explain why you would need to conjugate a drug to glucuronic acid and how glucuronides solve this problem.
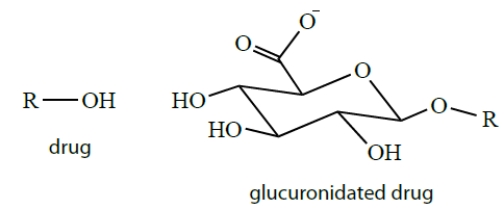
(Essay)
5.0/5  (35)
(35)
Arrange these compounds in order of increasing boiling point (lowest boiling point first).
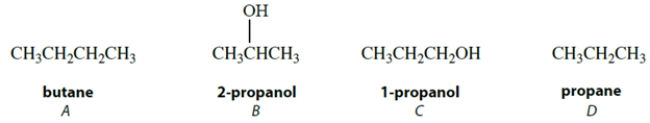
(Multiple Choice)
4.9/5  (38)
(38)
Draw the structure of 3-ethoxy-2-butanethiol (alternate name 3-ethoxybutane-2-thiol).
(Essay)
4.8/5  (30)
(30)
Which compound would form the strongest complex with potassium ion?
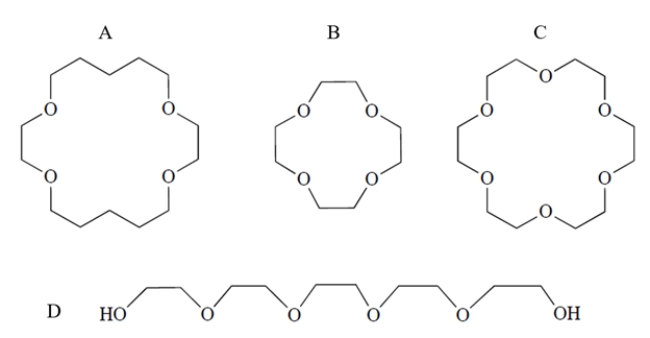
(Multiple Choice)
4.8/5  (38)
(38)
Select the structure of the compound with the highest boiling point.
(a)
(b)
(c)
(d)
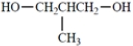
(Multiple Choice)
4.7/5  (37)
(37)
Provide a systematic name for the compound, including any stereochemical designations.
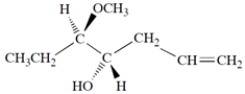
(Short Answer)
4.8/5  (32)
(32)
By circling three items below each structure, classify each of the following solvents as polar or apolar, donor or nondonor, protic or aprotic.
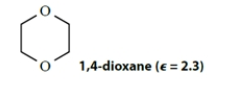
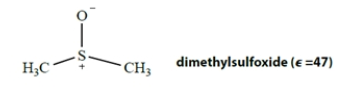 polar apolar donor nondonor protic aprotic polar apolar donor nondonor protic aprotic
polar apolar donor nondonor protic aprotic polar apolar donor nondonor protic aprotic
(Essay)
4.9/5  (36)
(36)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)