Deck 5: Addition Reactions of Alkenes and Alkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 5: Addition Reactions of Alkenes and Alkynes
1
Complete the reaction by giving the missing reactant.
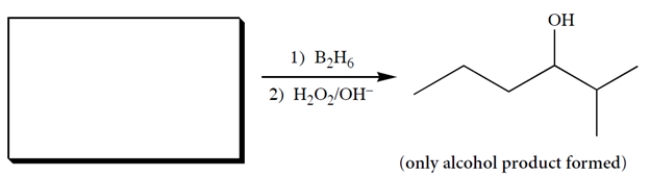

2
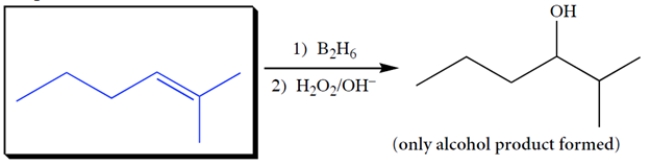
Complete the reaction by giving the missing product.

3
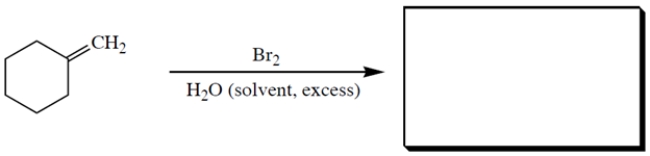
Complete the reaction by providing the missing starting material.
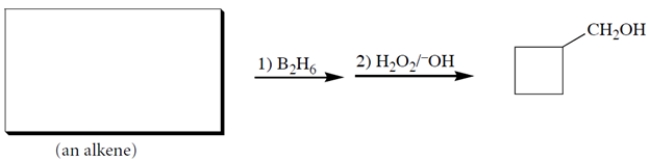

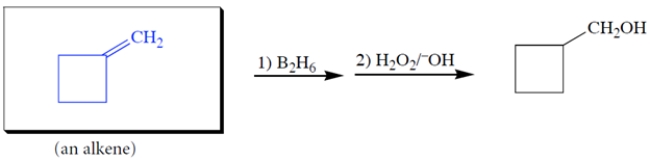
4
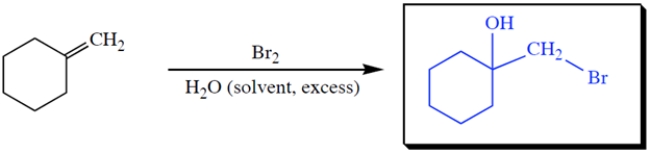
Complete the reaction by giving the missing product.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
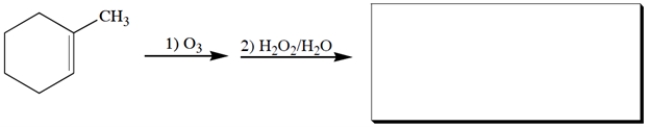
5
Complete the reaction by giving the missing product.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
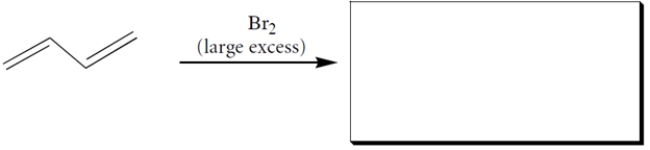
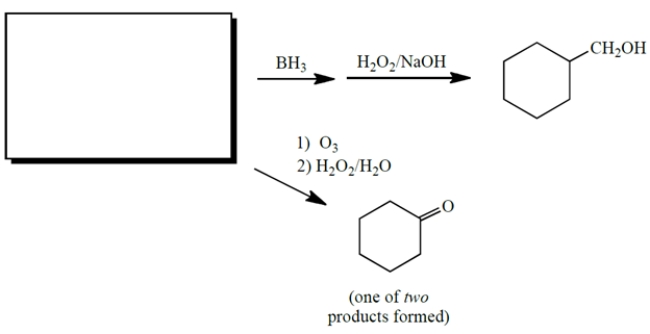
6
Complete the reactions by giving the missing starting material, which is the same for both reactions.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
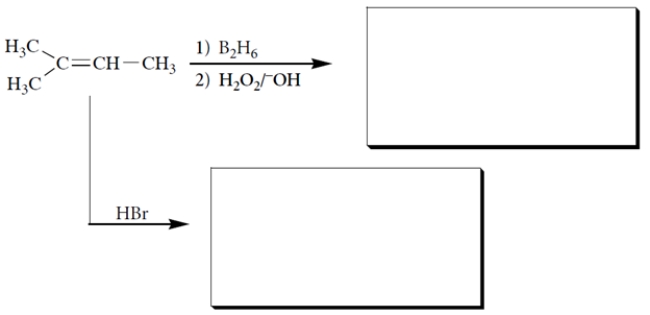
7
Complete the reactions by giving the structures of the missing organic products. (By-products are not shown.)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Complete the reaction by giving the missing starting material.
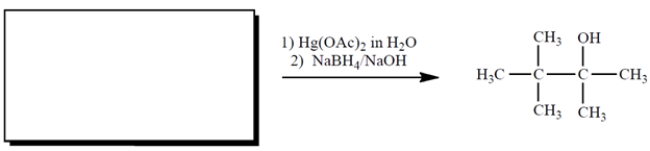
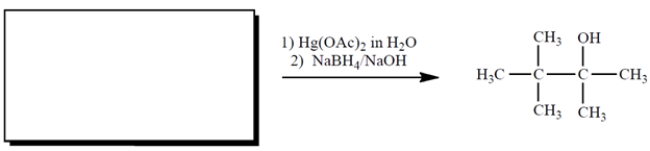

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the scheme.
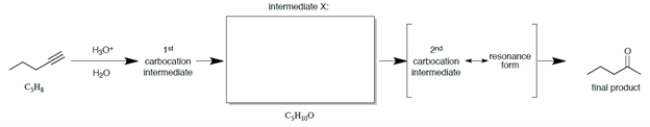 a. Draw the structure of intermediate X, which has the formula shown under in the box provided.
a. Draw the structure of intermediate X, which has the formula shown under in the box provided.
b. What special kind of functional group is present in intermediate X?
c. What special kind of functional group is present in the product?
 a. Draw the structure of intermediate X, which has the formula shown under in the box provided.
a. Draw the structure of intermediate X, which has the formula shown under in the box provided.b. What special kind of functional group is present in intermediate X?
c. What special kind of functional group is present in the product?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Complete the reaction by giving the missing starting material.
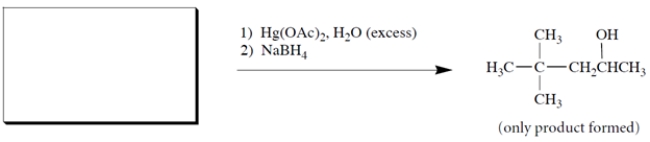
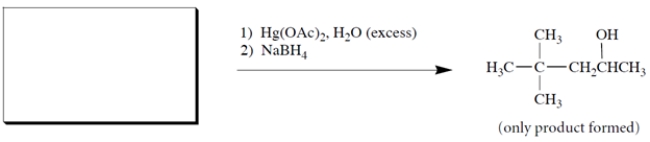

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Give the structure of the enol intermediate in the hydration of 1-pentyne.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
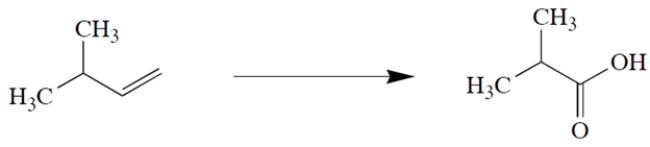
12
Identify the reagents needed to transform isopentene to the product shown.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
A student allowed an alcohol, 4-penten-1-ol, to react with Br2, expecting to observe a simple addition of Br2 to the double bond. Instead, she isolated a compound X that proved to have the formula C5H9OBr. The reaction mixture from which X was isolated was very acidic.
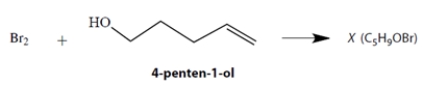 Using mechanistic reasoning, including the curved-arrow notation, propose a structure for compound X. Show your logic at every step of the way! Your answer should explain why the reaction mixture became acidic.
Using mechanistic reasoning, including the curved-arrow notation, propose a structure for compound X. Show your logic at every step of the way! Your answer should explain why the reaction mixture became acidic.
 Using mechanistic reasoning, including the curved-arrow notation, propose a structure for compound X. Show your logic at every step of the way! Your answer should explain why the reaction mixture became acidic.
Using mechanistic reasoning, including the curved-arrow notation, propose a structure for compound X. Show your logic at every step of the way! Your answer should explain why the reaction mixture became acidic.
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Complete the reaction by providing the structure of the missing product. (Ignore byproducts.)
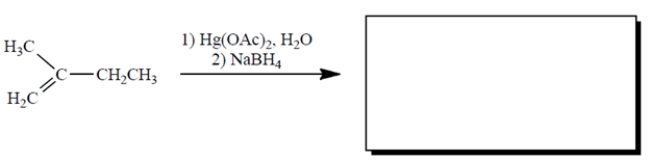


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Complete the reaction by providing the structure of the missing starting material.
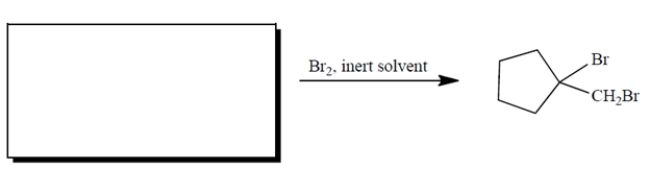


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Complete the reaction by providing the structure of the missing starting material.
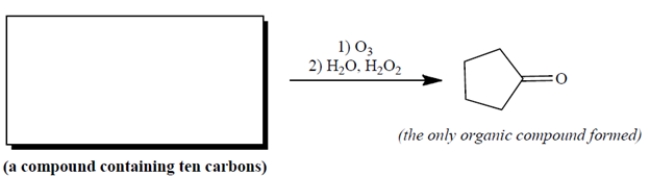


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Consider the reaction, in which X is a stable, mercury-containing compound (not a reactive intermediate). Compound Y does not react with either Br2, H2/catalyst, or ozone.
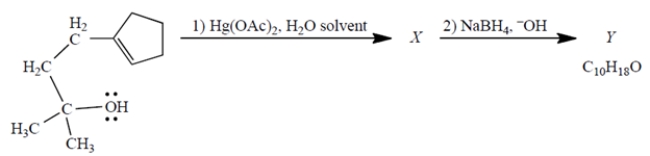 a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
b. Use mechanistic reasoning to deduce the structure of X. Use the curved-arrow notation; show all relevant unshared pairs and charges. Then, using what you know about step (2)-no mechanisms!-deduce the structure of the product Y.
 a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.b. Use mechanistic reasoning to deduce the structure of X. Use the curved-arrow notation; show all relevant unshared pairs and charges. Then, using what you know about step (2)-no mechanisms!-deduce the structure of the product Y.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Complete the reaction by providing a starting material with formula C10H16 that can give only the product shown at the right.
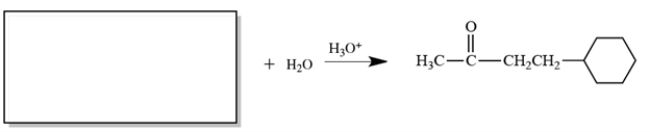


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Draw the structures of three enols that would be spontaneously converted into the following ketone. (Don't forget stereoisomers.)
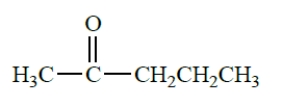
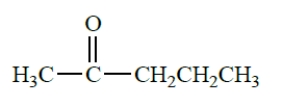

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Complete the reaction by providing the structure of the missing product.
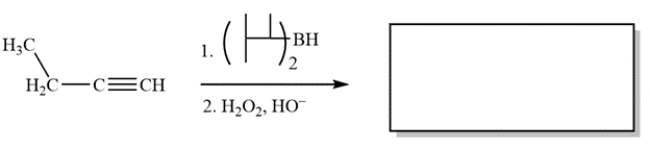


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Complete the reaction by providing the structure of the missing starting material.
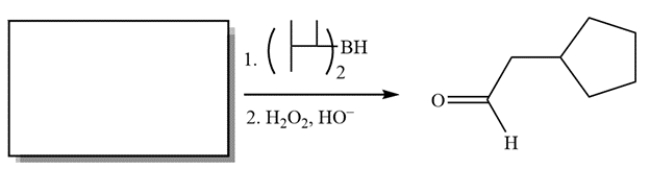


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Compare the results of hydroboration-oxidation and Hg2+-catalyzed hydration for 2-butyne versus 1-butyne. For which is the result of the two reactions the same and which results are different?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Devise a synthetic route for this conversion.
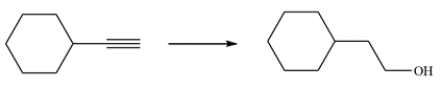


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Devise a synthetic route for this conversion.
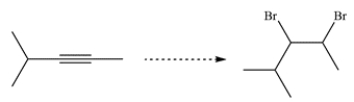


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
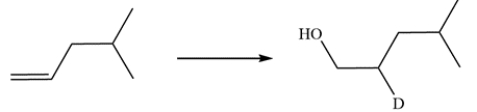
25
Devise a synthetic route for this conversion. Deuterium can be introduced by replacing the hydrogen in common reagents with its deuterium analog.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


