Exam 5: Addition Reactions of Alkenes and Alkynes
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
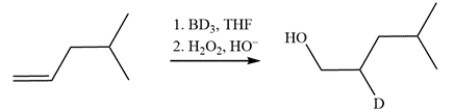
Devise a synthetic route for this conversion. Deuterium can be introduced by replacing the hydrogen in common reagents with its deuterium analog.
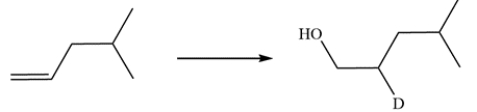
Free
(Essay)
4.7/5  (31)
(31)
Correct Answer:
This transformation is simply a hydroboration-oxidation reaction where the source of deuterium is BD3 (replacing BH3). The deuterium adds to the more substituted alkene carbon while the hydroxy group will end up on the less substituted alkene carbon.
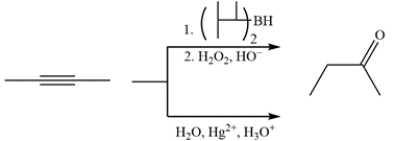
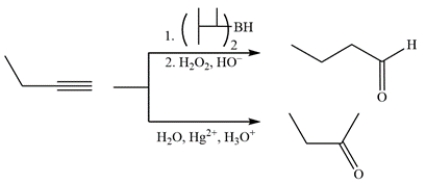
Compare the results of hydroboration-oxidation and Hg2+-catalyzed hydration for 2-butyne versus 1-butyne. For which is the result of the two reactions the same and which results are different?
Free
(Essay)
4.8/5  (44)
(44)
Correct Answer:
2-Butyne is a symmetrical alkyne and both reactions will give the same ketone product.
 1-Butyne is a terminal alkyne and will give an aldehyde using hydroboration-oxidation and a ketone using Hg2+-catalyzed hydration.
1-Butyne is a terminal alkyne and will give an aldehyde using hydroboration-oxidation and a ketone using Hg2+-catalyzed hydration.
Draw the structures of three enols that would be spontaneously converted into the following ketone. (Don't forget stereoisomers.)
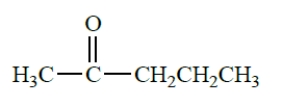
Free
(Essay)
4.8/5  (27)
(27)
Correct Answer:
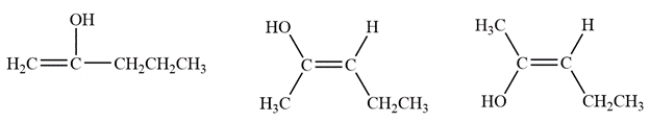
The enols are formed by removing an alpha proton from each side of the ketone and forming a pi bond. The three enols are
A student allowed an alcohol, 4-penten-1-ol, to react with Br2, expecting to observe a simple addition of Br2 to the double bond. Instead, she isolated a compound X that proved to have the formula C5H9OBr. The reaction mixture from which X was isolated was very acidic.
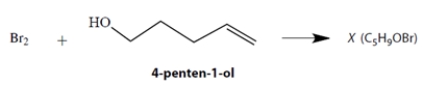 Using mechanistic reasoning, including the curved-arrow notation, propose a structure for compound X. Show your logic at every step of the way! Your answer should explain why the reaction mixture became acidic.
Using mechanistic reasoning, including the curved-arrow notation, propose a structure for compound X. Show your logic at every step of the way! Your answer should explain why the reaction mixture became acidic.
(Essay)
4.7/5  (38)
(38)
Complete the reaction by providing the structure of the missing product.
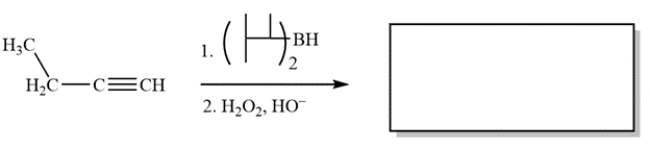
(Essay)
4.8/5  (30)
(30)
Give the structure of the enol intermediate in the hydration of 1-pentyne.
(Essay)
4.9/5  (36)
(36)
Consider the reaction, in which X is a stable, mercury-containing compound (not a reactive intermediate). Compound Y does not react with either Br2, H2/catalyst, or ozone.
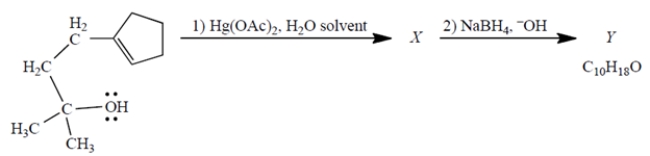 a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
b. Use mechanistic reasoning to deduce the structure of X. Use the curved-arrow notation; show all relevant unshared pairs and charges. Then, using what you know about step (2)-no mechanisms!-deduce the structure of the product Y.
a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
b. Use mechanistic reasoning to deduce the structure of X. Use the curved-arrow notation; show all relevant unshared pairs and charges. Then, using what you know about step (2)-no mechanisms!-deduce the structure of the product Y.
(Essay)
4.9/5  (38)
(38)
Identify the reagents needed to transform isopentene to the product shown.
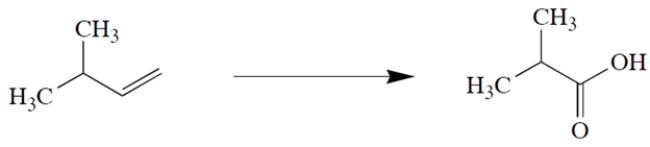
(Short Answer)
4.8/5  (33)
(33)
Complete the reaction by providing the structure of the missing starting material.
(Essay)
4.9/5  (31)
(31)
Complete the reaction by providing the structure of the missing product. (Ignore byproducts.)
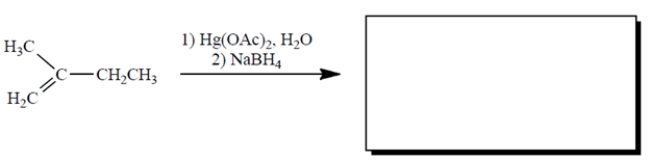
(Essay)
4.8/5  (34)
(34)
Complete the reactions by giving the missing starting material, which is the same for both reactions.
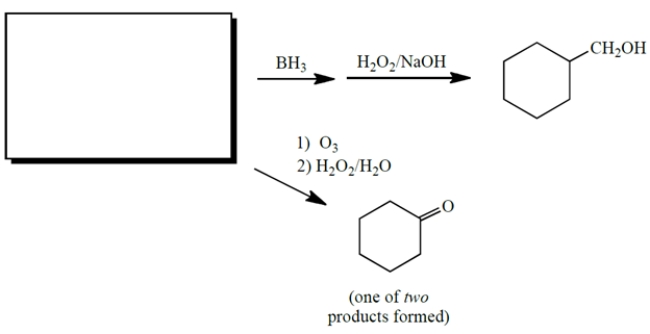
(Essay)
4.9/5  (38)
(38)
Consider the scheme.
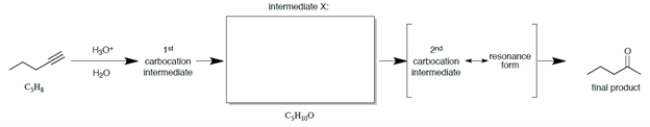 a. Draw the structure of intermediate X, which has the formula shown under in the box provided.
b. What special kind of functional group is present in intermediate X?
c. What special kind of functional group is present in the product?
a. Draw the structure of intermediate X, which has the formula shown under in the box provided.
b. What special kind of functional group is present in intermediate X?
c. What special kind of functional group is present in the product?
(Essay)
4.7/5  (29)
(29)
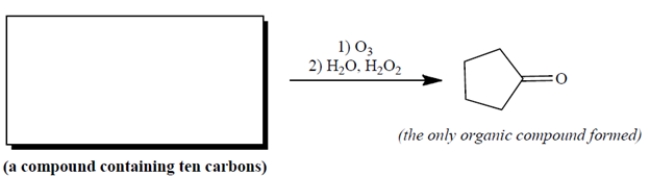
Complete the reaction by providing a starting material with formula C10H16 that can give only the product shown at the right.
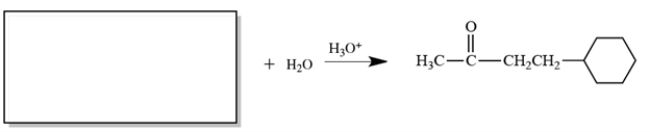
(Essay)
4.8/5  (43)
(43)
Complete the reaction by providing the structure of the missing starting material.
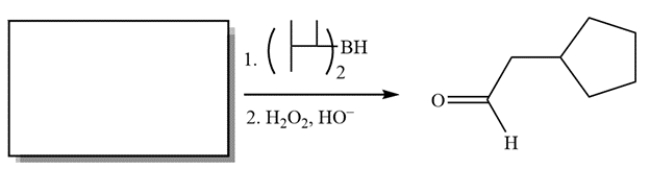
(Essay)
4.8/5  (38)
(38)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)