Deck 28: Pericyclic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 28: Pericyclic Reactions
1
Classify the reaction:
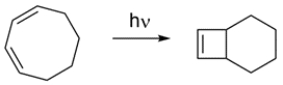
A) electrocyclic
B) cycloaddition
C) sigmatropic reaction

A) electrocyclic
B) cycloaddition
C) sigmatropic reaction
A
2
Classify the reaction:
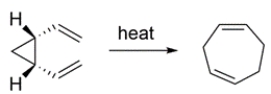
A) electrocyclic
B) cycloaddition
C) sigmatropic reaction

A) electrocyclic
B) cycloaddition
C) sigmatropic reaction
C
3
Classify the reaction:
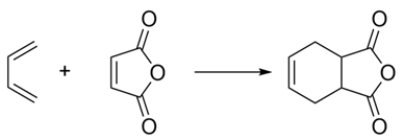
A) electrocyclic
B) cycloaddition
C) sigmatropic reaction

A) electrocyclic
B) cycloaddition
C) sigmatropic reaction
B
4
Consider the carbocation:
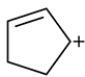 a. How many pi molecular orbitals are there?
a. How many pi molecular orbitals are there?
b. Classify each MO as symmetric or antisymmetric.
c. Which MOs are bonding and antibonding?
d. Which MOs are the HOMO and LUMO?
 a. How many pi molecular orbitals are there?
a. How many pi molecular orbitals are there?b. Classify each MO as symmetric or antisymmetric.
c. Which MOs are bonding and antibonding?
d. Which MOs are the HOMO and LUMO?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
Sketch the molecular orbitals for 1,3-pentadiene. Fill in the number of electrons in each molecular orbital, then identify the HOMO and LUMO. How many nodes are in each molecular orbital?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
Without drawing a molecular orbital diagram, consider the structure and identify
a. the number of pi electrons.
b. which molecular orbitals will be occupied.
c. which molecular orbitals are symmetric.
d. which molecular orbital is the HOMO.
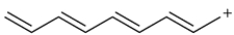
a. the number of pi electrons.
b. which molecular orbitals will be occupied.
c. which molecular orbitals are symmetric.
d. which molecular orbital is the HOMO.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
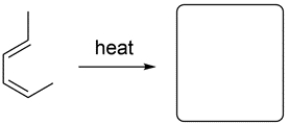
Given the starting material and conditions, identify the type of pericyclic reaction, the number of electrons involved in the reaction, and draw the major organic product. Clearly show stereochemistry, if applicable.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
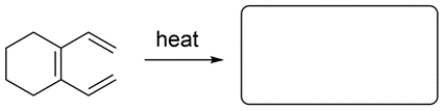
Given the starting material and conditions, identify the type of pericyclic reaction, the number of electrons involved in the reaction, and draw the major organic product. Clearly show stereochemistry, if applicable.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Which conditions should you use to perform the reaction and why?
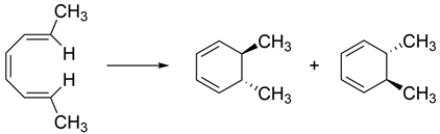
A) heat because the reaction is conrotatory
B) light (h) because the reaction is conrotatory
C) heat because the reaction is disrotatory
D) light (h) because the reaction is disrotatory

A) heat because the reaction is conrotatory
B) light (h) because the reaction is conrotatory
C) heat because the reaction is disrotatory
D) light (h) because the reaction is disrotatory

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
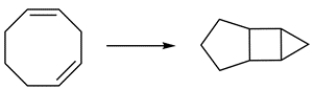
10
Which conditions should you use to perform the reaction and why?


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
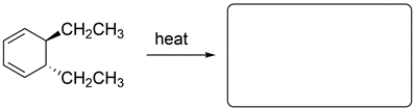
11
Predict the major organic product and show stereochemistry where relevant.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Two ethylene molecules can undergo a [2+2] cycloaddition, but only under photochemical conditions. Explain this difference in reactivity.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
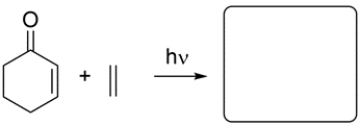
13
Given the starting material and conditions, identify the type of pericyclic reaction, the number of electrons involved in the reaction, and draw the major organic product. Clearly show stereochemistry, if applicable.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
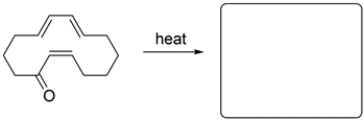
14
Predict the major organic product for the reaction.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Classify the sigmatropic reaction with bracketed numbers.
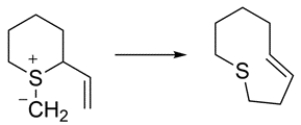


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Classify the sigmatropic reaction with bracketed numbers.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Given the starting material and conditions, identify the type of pericyclic reaction, the number of electrons involved in the reaction, and draw the major organic product. Clearly show stereochemistry, if applicable.
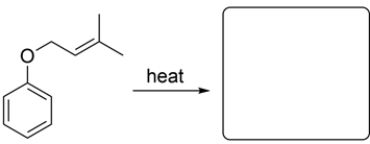


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Predict the major organic products in the boxes and show stereochemistry where relevant.
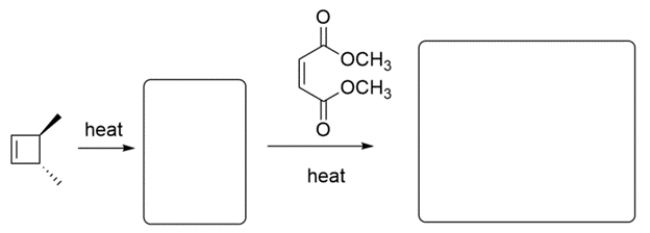


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Draw curved-arrow mechanism showing how this transformation occurs.
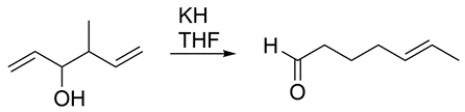


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Outline a synthesis for this transformation. A key step should be a [2,2] or [4,2] cycloaddition.
![Outline a synthesis for this transformation. A key step should be a [2,2] or [4,2] cycloaddition.](https://storage.examlex.com/TBMC1048/11edaded_7d21_9282_a31a_8d3fdf63afbc_TBMC1048_00.jpg)
![Outline a synthesis for this transformation. A key step should be a [2,2] or [4,2] cycloaddition.](https://storage.examlex.com/TBMC1048/11edaded_7d21_9282_a31a_8d3fdf63afbc_TBMC1048_00.jpg)

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
This transformation can occur through two pericyclic reactions. Outline a synthesis for the transformation.
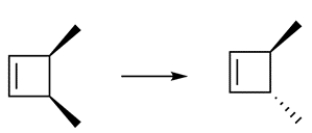


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Deduce the starting materials needed to synthesize this compound.
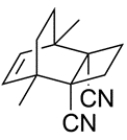


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Outline a synthetic sequence to give this transformation. Hint: A sigmatropic rearrangement is a key step.
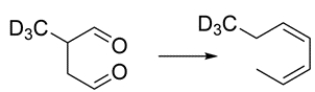


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
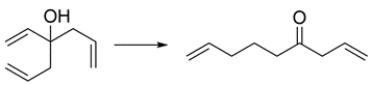
Draw a curved-arrow mechanism showing how the alcohol is transformed to the ketone.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
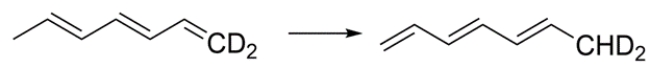
Give the product for a [1,5] deuterium shift for this structure:
![Give the product for a [1,5] deuterium shift for this structure:](https://storage.examlex.com/TBMC1048/11edaded_7d21_e0ac_a31a_8fce8c1e7038_TBMC1048_00.jpg)
![Give the product for a [1,5] deuterium shift for this structure:](https://storage.examlex.com/TBMC1048/11edaded_7d21_e0ac_a31a_8fce8c1e7038_TBMC1048_00.jpg)

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


