Exam 28: Pericyclic Reactions
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Two ethylene molecules can undergo a [2+2] cycloaddition, but only under photochemical conditions. Explain this difference in reactivity.
Free
(Essay)
5.0/5  (29)
(29)
Correct Answer:
In ethylene, the HOMO and LUMO have different phases. The phases do not overlap, so the reaction is forbidden.
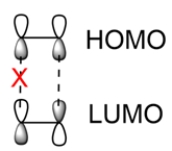 When ethylene undergoes photochemical conditions, one of the electrons can be promoted to a higher energy level, so the HOMO now has p orbitals with opposite phases. This matches the phases of the LUMO, so the photochemical reaction is allowed.
When ethylene undergoes photochemical conditions, one of the electrons can be promoted to a higher energy level, so the HOMO now has p orbitals with opposite phases. This matches the phases of the LUMO, so the photochemical reaction is allowed.
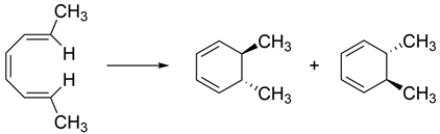
Which conditions should you use to perform the reaction and why?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
B
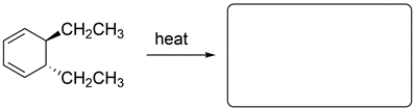
Predict the major organic product and show stereochemistry where relevant.
Free
(Essay)
4.7/5  (35)
(35)
Correct Answer:
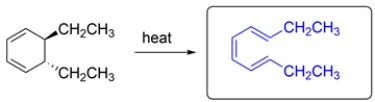 The reaction is a ring-opening electrocyclic reaction. There are six electrons involved in the reaction and it is heated, so the reaction will be disrotatory. The two ethyl substituents can be either cis/cis or trans/trans. The cis/cis would be highly hindered, so the trans/trans isomer predominates.
The reaction is a ring-opening electrocyclic reaction. There are six electrons involved in the reaction and it is heated, so the reaction will be disrotatory. The two ethyl substituents can be either cis/cis or trans/trans. The cis/cis would be highly hindered, so the trans/trans isomer predominates.
Given the starting material and conditions, identify the type of pericyclic reaction, the number of electrons involved in the reaction, and draw the major organic product. Clearly show stereochemistry, if applicable.
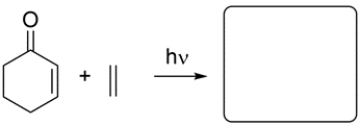
(Essay)
4.8/5  (35)
(35)
Given the starting material and conditions, identify the type of pericyclic reaction, the number of electrons involved in the reaction, and draw the major organic product. Clearly show stereochemistry, if applicable.
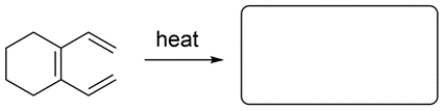
(Essay)
4.9/5  (25)
(25)
Sketch the molecular orbitals for 1,3-pentadiene. Fill in the number of electrons in each molecular orbital, then identify the HOMO and LUMO. How many nodes are in each molecular orbital?
(Essay)
4.7/5  (32)
(32)
Outline a synthetic sequence to give this transformation. Hint: A sigmatropic rearrangement is a key step.
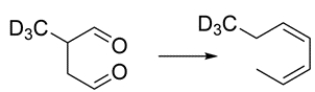
(Essay)
4.8/5  (43)
(43)
This transformation can occur through two pericyclic reactions. Outline a synthesis for the transformation.
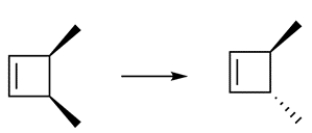
(Essay)
4.8/5  (27)
(27)
Consider the carbocation:
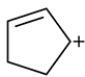 a. How many pi molecular orbitals are there?
b. Classify each MO as symmetric or antisymmetric.
c. Which MOs are bonding and antibonding?
d. Which MOs are the HOMO and LUMO?
a. How many pi molecular orbitals are there?
b. Classify each MO as symmetric or antisymmetric.
c. Which MOs are bonding and antibonding?
d. Which MOs are the HOMO and LUMO?
(Essay)
4.7/5  (29)
(29)
Predict the major organic products in the boxes and show stereochemistry where relevant.
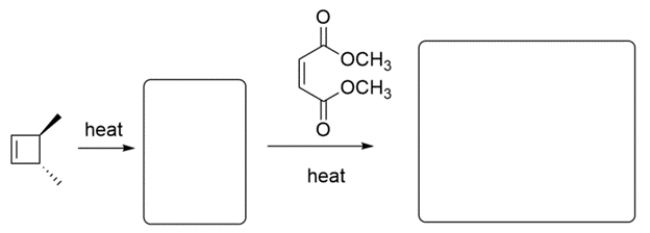
(Essay)
4.7/5  (35)
(35)
Given the starting material and conditions, identify the type of pericyclic reaction, the number of electrons involved in the reaction, and draw the major organic product. Clearly show stereochemistry, if applicable.
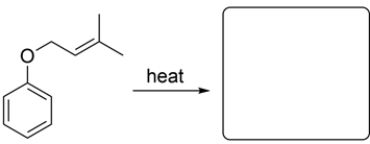
(Essay)
4.7/5  (32)
(32)
Outline a synthesis for this transformation. A key step should be a [2,2] or [4,2] cycloaddition.
![Outline a synthesis for this transformation. A key step should be a [2,2] or [4,2] cycloaddition.](https://storage.examlex.com/TBMC1048/11edaded_7d21_9282_a31a_8d3fdf63afbc_TBMC1048_00.jpg)
(Essay)
4.9/5  (47)
(47)
Draw a curved-arrow mechanism showing how the alcohol is transformed to the ketone.
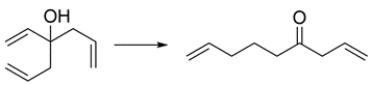
(Essay)
5.0/5  (30)
(30)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)