Deck 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids
1
Name the heterocycle.
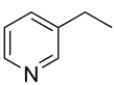

The heterocycle is 3-ethylpyridine.
2
Name the heterocycle.
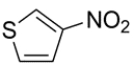

The heterocycle is 3-nitrothiophene.
3
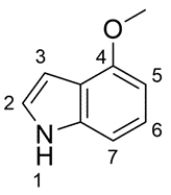
Draw a structure for each of the compounds.
a. 4-methoxyindole
b. thiophene-2-carboxylic acid
a. 4-methoxyindole
b. thiophene-2-carboxylic acid
a.
b.
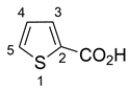

b.

4
Draw a structure for each of the compounds.
a. oxazole-5-carbaldehyde
b. 4-methoxypyridine
a. oxazole-5-carbaldehyde
b. 4-methoxypyridine

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
The structure of indole is shown below. Unlike most amines, indole is difficult to protonate. Its conjugate acid has a pKa of -3.5. Explain why indole is difficult to protonate.
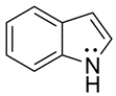


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
An imidazole ring is an important heterocycle found in the amino acid histidine. Draw an orbital configuration of imidazole, showing the 2p orbitals and electrons in each orbital. Use the diagram to explain which electrons are part of the aromatic system and which can be protonated in the presence of an acid.
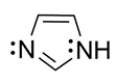


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
Purine is a bicyclic ring found in DNA. The structure for purine is shown below. For each lone pair of nitrogen, determine whether the lone pair resides on a 2p orbital or sp2 orbital.
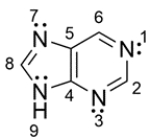


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
For each heterocycle, label as aromatic, anti-aromatic, or nonaromatic.
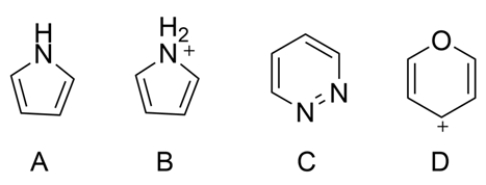


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Circle the nitrogen that is more basic and explain why.
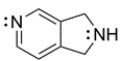


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Rank the reactivity of these compounds towards electrophilic aromatic substitution, from most reactive to least.
benzene, furan, thiophene, pyrrole, pyridine
benzene, furan, thiophene, pyrrole, pyridine

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Predict the major organic product for the reaction.
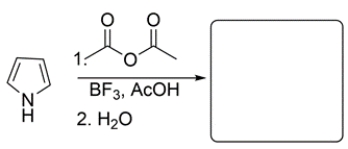


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Thiophene undergoes electrophilic aromatic substitution (EAS) with bromine to give two possible regioisomers, A and

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Outline a synthesis for the transformation:
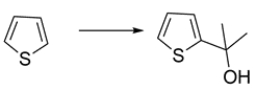


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Outline a synthesis for the transformation:



Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the missing reagent(s) for the transformation:
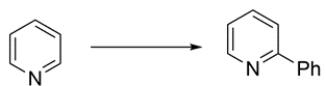


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
2-Halopyridines undergo substitution under milder conditions than the Chichibabin reaction. Write a curved-arrow mechanism for this substitution.
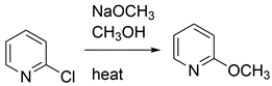


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Predict the major organic product for this synthetic sequence.
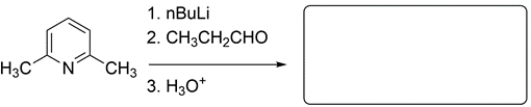


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
In biological systems, pyridoxal phosphate is a cofactor that aids in the decarboxylation of glutamic acid to 4-aminobutanoic acid. The key intermediate is shown below. Draw curved arrows to show the decarboxylation step.
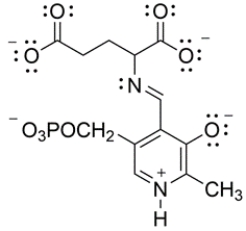


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the correct name or abbreviation of the given nucleoside or nucleotide:
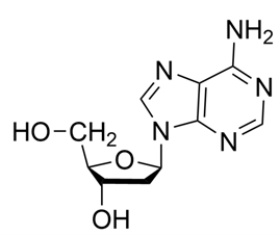
A) 2ʹ-deoxyadenosine
B) adenine
C) adenosine
D) dATP

A) 2ʹ-deoxyadenosine
B) adenine
C) adenosine
D) dATP

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Shown below are the two types of base pairs in DNA, with the bases in the same relative alignments that they are in DNA.
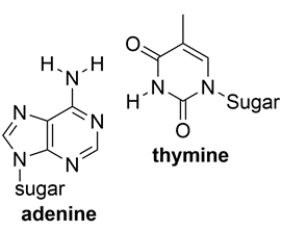
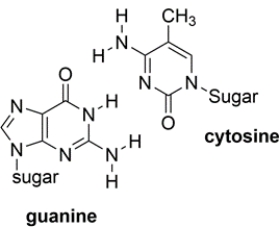 a. Show all the relevant hydrogen bonds with dotted lines and include the unshared pairs on the acceptor atoms, within each pair.
a. Show all the relevant hydrogen bonds with dotted lines and include the unshared pairs on the acceptor atoms, within each pair.
b. Which base pair has the strongest intermolecular interaction and why?

 a. Show all the relevant hydrogen bonds with dotted lines and include the unshared pairs on the acceptor atoms, within each pair.
a. Show all the relevant hydrogen bonds with dotted lines and include the unshared pairs on the acceptor atoms, within each pair.b. Which base pair has the strongest intermolecular interaction and why?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
The nitrogen mustard mustine is toxic to cells because it reacts with two DNA bases on opposite strands of the double helix. Representing the two reacting bases of DNA as R3N: and Rʹ3N: show the products of the reaction of mustine with DNA in a manner that would crosslink the two DNA strands. Your mechanism should account for the very high reactivity of mustine. Show all charges and unshared pairs.
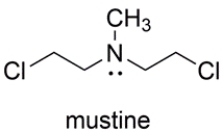


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Give the complementary sequence to the DNA strand.
5ʹ-TATGAC-3ʹ
5ʹ-TATGAC-3ʹ

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Give the complementary sequence to the DNA strand.
5ʹ-ACTGAC-3ʹ
5ʹ-ACTGAC-3ʹ

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
The heterocyclic base 7-aminopropargyl-7-deaza-2,6-diaminopurine has been incorporated into a short sequence of nucleotides. The structure is shown below. Compare it to the other nucleotides and determine which nucleotide base is it likely to pair with. Draw the matching nucleotide with hydrogen bonds as dotted lines.
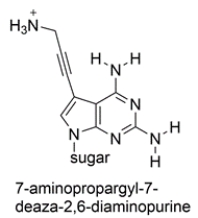


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
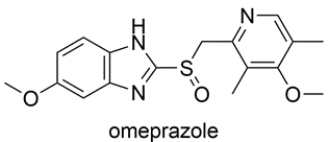
Omeprazole is a drug used for the treatment of acid-reflux disease. The structure is shown below. Highlight the aromatic heterocycles in the structure and name the heterocycles.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


