Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids
Identify the missing reagent(s) for the transformation:
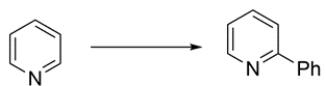
The pyridine ring can undergo nucleophilic aromatic substitution. An organolithium reagent, such as PhLi, will be used to form a carbon-carbon bond. An aqueous workup gives the desired substituted pyridine. The byproduct of the reaction is LiH.
For each heterocycle, label as aromatic, anti-aromatic, or nonaromatic.
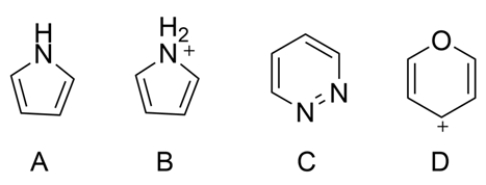
Start by adding lone pairs to each heteroatom.
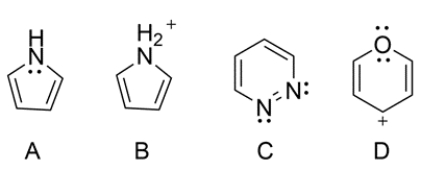 Compound A is aromatic, as the nitrogen contributes two electrons to the pi electron system, giving an overall count of six pi electrons. Compound B is nonaromatic, as it does not have a p orbital on the nitrogen and is sp3 hybridized. Compound C is aromatic, as the lone pairs on each nitrogen are located on an sp2 orbital and not part of the pi electron system. Compound D is aromatic, as one of the lone pairs on the oxygen contributes to the pi electron system of the ring, giving an overall count of six pi electrons.
Compound A is aromatic, as the nitrogen contributes two electrons to the pi electron system, giving an overall count of six pi electrons. Compound B is nonaromatic, as it does not have a p orbital on the nitrogen and is sp3 hybridized. Compound C is aromatic, as the lone pairs on each nitrogen are located on an sp2 orbital and not part of the pi electron system. Compound D is aromatic, as one of the lone pairs on the oxygen contributes to the pi electron system of the ring, giving an overall count of six pi electrons.
Outline a synthesis for the transformation:
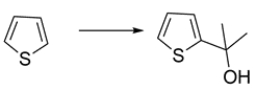
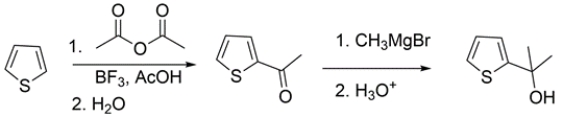 The first step is an electrophilic aromatic substitution to add the acetyl group. The second step is a Grignard addition to the ketone to give the tertiary alcohol.
The first step is an electrophilic aromatic substitution to add the acetyl group. The second step is a Grignard addition to the ketone to give the tertiary alcohol.
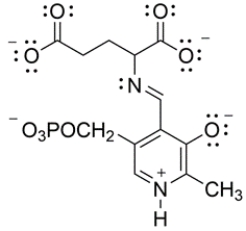
In biological systems, pyridoxal phosphate is a cofactor that aids in the decarboxylation of glutamic acid to 4-aminobutanoic acid. The key intermediate is shown below. Draw curved arrows to show the decarboxylation step.
Shown below are the two types of base pairs in DNA, with the bases in the same relative alignments that they are in DNA.
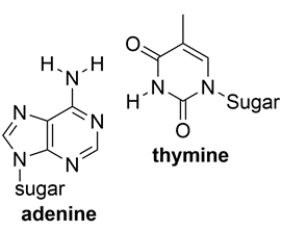
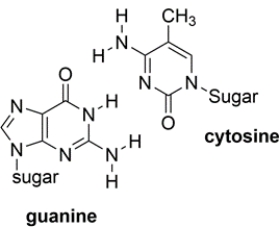 a. Show all the relevant hydrogen bonds with dotted lines and include the unshared pairs on the acceptor atoms, within each pair.
b. Which base pair has the strongest intermolecular interaction and why?
a. Show all the relevant hydrogen bonds with dotted lines and include the unshared pairs on the acceptor atoms, within each pair.
b. Which base pair has the strongest intermolecular interaction and why?
Draw a structure for each of the compounds.
a. oxazole-5-carbaldehyde
b. 4-methoxypyridine
Draw a structure for each of the compounds.
a. 4-methoxyindole
b. thiophene-2-carboxylic acid
Rank the reactivity of these compounds towards electrophilic aromatic substitution, from most reactive to least.
benzene, furan, thiophene, pyrrole, pyridine
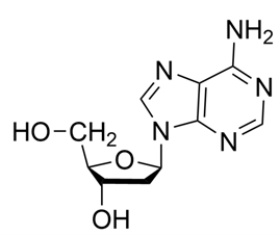
Identify the correct name or abbreviation of the given nucleoside or nucleotide:
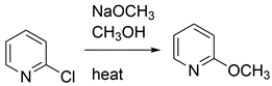
2-Halopyridines undergo substitution under milder conditions than the Chichibabin reaction. Write a curved-arrow mechanism for this substitution.
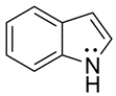
The structure of indole is shown below. Unlike most amines, indole is difficult to protonate. Its conjugate acid has a pKa of -3.5. Explain why indole is difficult to protonate.
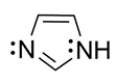
An imidazole ring is an important heterocycle found in the amino acid histidine. Draw an orbital configuration of imidazole, showing the 2p orbitals and electrons in each orbital. Use the diagram to explain which electrons are part of the aromatic system and which can be protonated in the presence of an acid.
Thiophene undergoes electrophilic aromatic substitution (EAS) with bromine to give two possible regioisomers, A and
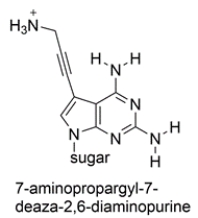
The heterocyclic base 7-aminopropargyl-7-deaza-2,6-diaminopurine has been incorporated into a short sequence of nucleotides. The structure is shown below. Compare it to the other nucleotides and determine which nucleotide base is it likely to pair with. Draw the matching nucleotide with hydrogen bonds as dotted lines.
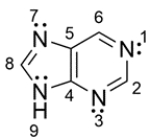
Purine is a bicyclic ring found in DNA. The structure for purine is shown below. For each lone pair of nitrogen, determine whether the lone pair resides on a 2p orbital or sp2 orbital.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)