Deck 20: The Chemistry of Carboxylic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 20: The Chemistry of Carboxylic Acids
1
Name the carboxylic acid.
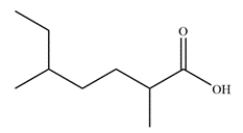

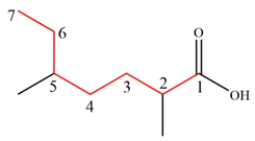
The longest chain containing the carboxylic acid is highlighted in red below. The name is 2,5-dimethylheptanoic acid.

2
Name the carboxylic acid using Greek letters, rather than numbers.
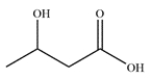

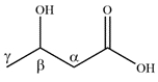
As in aldehydes and ketones, the carbons next to the functional group can be denoted by Greek letters. There are four carbons in the molecule, so the parent chain is butyric acid. The hydroxy group is on the beta carbon, so the name is -hydroxybutyric acid.

3
Give the IUPAC and common name for the dicarboxylic acid.
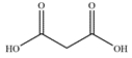

The dicarboxylic acid has the IUPAC name propanedioic acid and the common name, malonic acid.
4
Rank the functional groups in order of highest priority to lowest for nomenclature.
A) alcohol > aldehyde > carboxylic acid > thiol
B) aldehyde > carboxylic acid > thiol > alcohol
C) thiol > carboxylic acid > aldehyde > alcohol
D) carboxylic acid > aldehyde > alcohol > thiol
A) alcohol > aldehyde > carboxylic acid > thiol
B) aldehyde > carboxylic acid > thiol > alcohol
C) thiol > carboxylic acid > aldehyde > alcohol
D) carboxylic acid > aldehyde > alcohol > thiol

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
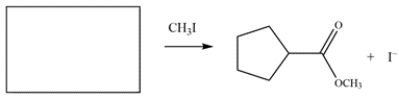
5
Draw the structure of the nucleophile (including formal charges) that would give the product on the right.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
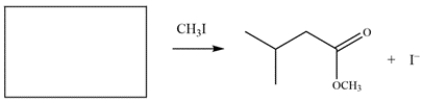
6
Draw the structure of the nucleophile (including formal charges) that would give the product on the right.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
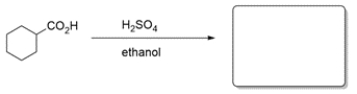
7
Predict the major organic product for the reaction. If you believe no reaction will occur, write NR.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Outline a synthesis to prepare the ester from cyclohexene.
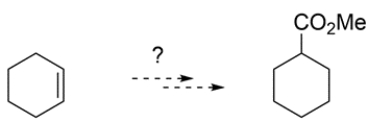


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Provide structures for products A-D. The 1H NMR spectrum of D is given.
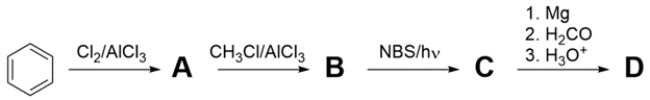
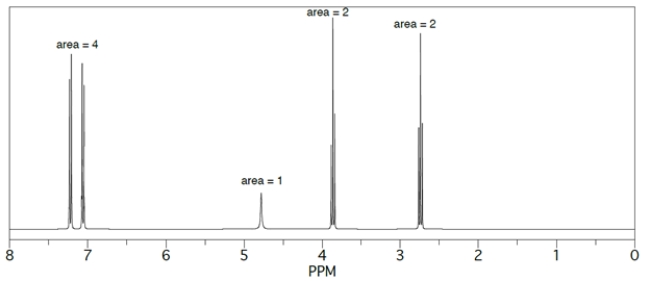
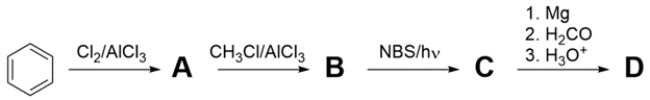


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Predict the major organic product for the reaction. If you believe no reaction would occur, write NR.
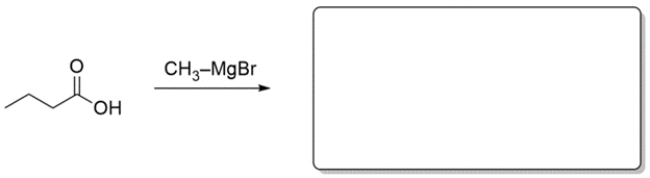


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Which compound decarboxylates fastest?
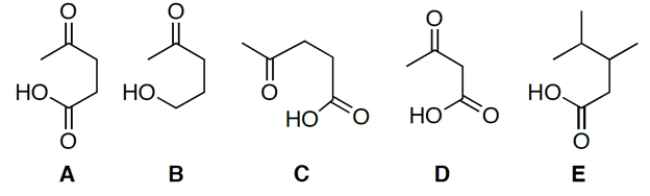
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Provide a detailed, arrow-pushing mechanism for this transformation. Show all reactive intermediates and all proton transfer steps. At some point in your answer, show the two most important resonance structures for diazomethane, CH2N2, and identify the gas that is produced in the reaction.



Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Which side is favored at equilibrium?

A) the left
B) the right
C) need to know pKa values of the individual acids before estimating
D) the equilibrium depends on the temperature of the measurement, and thus can't be known
E) neither, Keq = 1

A) the left
B) the right
C) need to know pKa values of the individual acids before estimating
D) the equilibrium depends on the temperature of the measurement, and thus can't be known
E) neither, Keq = 1

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the carboxylic acids would not decarboxylate (lose CO2) upon warming?
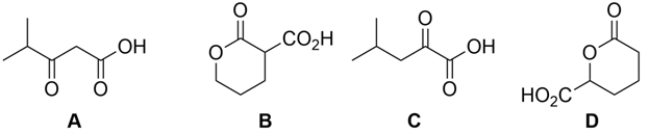
A) compound A
B) compound B
C) compound C
D) compound D
E) neither compound C nor compound D would decarboxylate upon warming

A) compound A
B) compound B
C) compound C
D) compound D
E) neither compound C nor compound D would decarboxylate upon warming

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
A student has synthesized two isomers, A and B, but neglected to label the flasks. Suggest a spectroscopy method the student could use to quickly differentiate between them and explain what feature they would be looking for.
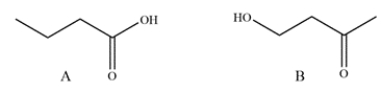
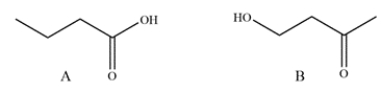

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
A compound with the formula C9H10O2 has this 1H NMR spectra. Deduce the structure.
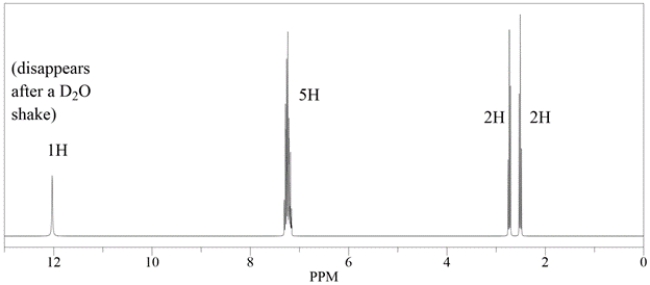


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Which statement is true of soaps?
A) Soap is made by reduction of a fatty acid.
B) Soap is positively charged.
C) Soap has a long hydrocarbon tail and ionic head group.
D) Soap molecules form micelles, where the polar head groups are on the inside of the ball and the tails are on the outside.
A) Soap is made by reduction of a fatty acid.
B) Soap is positively charged.
C) Soap has a long hydrocarbon tail and ionic head group.
D) Soap molecules form micelles, where the polar head groups are on the inside of the ball and the tails are on the outside.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Outline a multistep synthesis to perform the transformation.
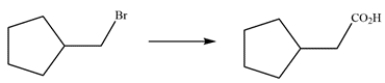


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Outline a multistep synthesis of the ketone from the carboxylic acid.
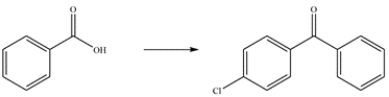


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
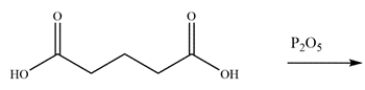
20
Predict the major organic product for the reaction.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
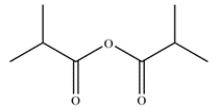
21
Outline a one-step synthesis of the anhydride.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
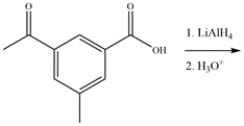
22
Predict the major organic product for the reaction.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
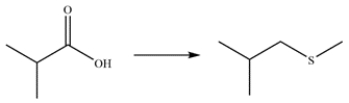
23
Outline a multistep synthesis of the transformation.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
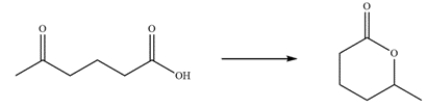
24
Outline a multistep synthesis of the transformation.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
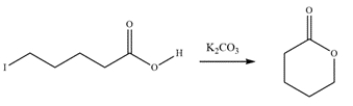
25
Draw a curved arrow mechanism for the esterification.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


